QuantiFast Multiplex PCR Kits
使用序列特异性探针进行快速的多重两步法RT-PCR,适用于基因表达分析
使用序列特异性探针进行快速的多重两步法RT-PCR,适用于基因表达分析

Cat. No. / ID: 204654

Cat. No. / ID: 204756
应用QuantiFast Multiplex PCR Kit可在单管内通过多重两步法real-time PCR对多至4个cDNA进行快速、可靠的定量检测。Q-Bond和优化的预混液促进快速多重real-time PCR的进行,适用于快速或标准PCR仪。即用型预混液中的热启动酶和独特的PCR缓冲液可确保在所有real-time PCR仪上进行灵敏的qPCR,无需优化。有两种规格的试剂盒:QuantiFast Multiplex PCR Kit适用于需要ROX染料进行荧光信号校准的PCR仪,QuantiFast Multiplex PCR +R Kit适用于其他所有PCR仪。为便于使用,预混液可保存在2–8°C。
随QuantiFast Multiplex PCR Kit提供的特制的预混液可快速构建多重反应,得到的多重PCR反应结果可与单重PCR反应结果相媲美(参见" Comparable results in triplex and singleplex PCR")。该试剂盒可清楚区分模板量的细微差别。即使模板量仅有两倍的差别,该试剂盒也能精确定量丰度差别较大的2个靶基因(参见" Linear CT values over twofold decreases in template")。
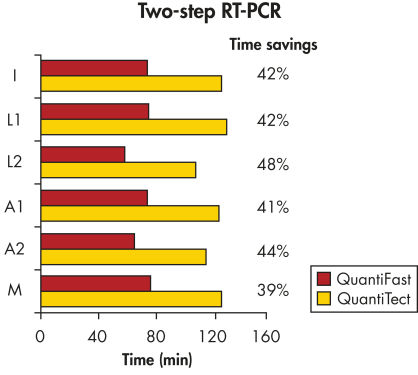
QuantiFast Multiplex PCR Kits可节省多达50%的PCR反应时间,让您更快获得结果(参见" Significantly reduced PCR times")。只需60分钟,即可检测到低至10个拷贝的靶基因(参见" Sensitive duplex PCR and a wide dynamic range")。您可极大地提高样本通量或与其它使用者有效地共享PCR仪。该试剂盒可对多达4个靶基因进行快速、多重real-time PCR,且不会影响PCR效果(参见" Uncompromised sensitivity")。
QuantiFast Multiplex PCR Kits可在标准或快速PCR仪上快速、灵敏的获得反应结果,无需优化(参见" QIAGEN multiplex kits")。特制的快速PCR缓冲液含有新型Q-Bond成分,可明显缩短变性、退火和延伸的时间(参见" Fast primer annealing")。
在同一个反应中同时扩增对照和靶基因,而非分开反应,减少了操作误差,提高了检测的可靠性。QuantiFast Multiplex PCR Buffer 含有平衡配比的K+和NH4+以及独特的MP因子,二者可促进引物、探针与核酸模板的稳定高效结合,确保高效的PCR反应(参见" Unique PCR buffer")此外,HotStarTaq Plus DNA Polymerase要求严谨的热启动,可阻止非特异性产物的形成。
| 成分 | 特点 | 优势 |
|---|---|---|
| HotStarTaq Plus DNA Polymerase | 95ºC加热5分钟活化 | 在室温进行qPCR反应体系构建 |
| QuantiFast Multiplex PCR Buffer | 平衡的NH4+和K+离子配比 | 引物探针的特异性结合确保获得可靠结果 |
| 合成的MP因子 | 在单管中可靠的对至多4个基因进行多重分析 | |
| 独特的Q-Bond添加剂 | PCR运行时间缩短,更快获得结果,一天内可完成更多PCR反应 | |
| ROX染料† | 对Applied Biosystems和Agilent PCR仪进行荧光信号的校准 | 对需要ROX染料的PCR仪进行校准,不影响PCR反应结果 |
QuantiFast Multiplex PCR Kit含有即用型预混液,无需优化反应及循环条件。只需将cDNA和引物探针对加入预混液中,按照操作手册进行实验,即可快速获得可靠的结果,适用于所有real-time PCR仪。试剂盒提供两种形式:预混液中含有或不含荧光校正用的ROX染料,可用于所有PCR仪(参见下表)。由于ROX浓度经优化,即使是低拷贝数也可通过数据自动分析进行检测。
| ROX染料 | 试剂盒 | 适用的PCR仪 |
|---|---|---|
| 混合在预混液中 | QuantiFast Multiplex PCR Kit | 除Applied Biosystems 7500外的所有Applied Biosystems仪器 |
| 单独装在管中 | QuantiFast Multiplex PCR +R Kit | Applied Biosystems 7500、Bio-Rad、Cepheid、Eppendorf、QIAGEN、Roche、Agilent和其他供应商的PCR仪 |
为获得最佳的两步法real-time RT-PCR结果,我们建议使用QuantiTect Reverse Transcription Kit合成cDNA。该试剂盒可在20分钟进行快速cDNA合成,并去除基因组DNA污染。
QuantiFast Probe Assays利用序列特异性水解探针检测技术,是适合全基因组的预制试剂。 随QuantiFast Multiplex PCR Kit提供,可确保在双染两步法real-time RT-PCR反应中获得可靠结果。
QuantiFast Multiplex PCR Kit可用在各种real-time PCR仪上对cDNA进行多重基因表达分析,适用仪器包括Agilent、Applied Biosystems、Bio-Rad、Cepheid、Eppendorf和Roche的PCR仪。在Rotor-Gene Q实时荧光定量PCR分析仪或其他Rotor-Gene PCR仪上使用时,我们推荐使用专为快速PCR研发的Rotor-Gene Multiplex PCR Kit。
| Features | Specifications |
|---|---|
| Applications | Quantification of cDNA or genomic DNA targets in a multiplex real-time PCR |
| Thermal cycler | Real-time cyclers dedicated for multiplex PCR (e.g., most Applied Biosystems real-time PCR cyclers, Roche LightCycler® 480, iCycler iQ®, Rotor-Gene) |
| Real-time or endpoint | Real-time |
| Sample/target type | cDNA, DNA |
| SYBR Green I or sequence-specific probes | Sequence-specific probes |
| Reaction type | Real-time two-step RT-PCR |
| Single or multiplex | Multiplex |
| With or without ROX | Available with ROX in master mix and with ROX as separate vial |