Products
特点
- 使用一个 AAV 裂解试剂盒和一种方案使当前工作流程的标准化和质量控制 (Quality Control, QC)成为可能
- 对多种血清型(如 AAV 血清型)的病毒滴度进行一致且可重复的测定
- 与 QIAcuity Cell and Gene Therapy (CGT) dPCR Assay 相结合的完整工作流程,可在 QIAcuity Digital PCR System 上实现 AAV 滴度的准确测定
产品详情
腺相关病毒是在基因治疗应用领域广泛使用的一种病毒载体。然而,病毒载体的生产和纯化过程需要精密的质量控制,以确保临床研究或患者治疗期间给药的安全性和可靠性。准确定量载体滴度以及确定污染的能力是安全且有效的基于 AAV 的基因治疗的关键。
随着 CGT Viral Vector Lysis Kit(包含用于 100 或 1000 次反应的试剂)的推出,我们现在拥有了一个经过优化的和标准化的病毒载体裂解工作流程,可以准确且精密地测定病毒滴度。该试剂盒也可用于腺病毒裂解。
绩效
我们提供了从进行 AAV 裂解到在细胞裂解液中定量病毒滴度的完整工作流程。CGT Viral Vector Lysis Kit 及其改进的配方可对最终滴度进行一致、稳健且准确的测定。它与 QIAcuity Cell and Gene Therapy dPCR Assay、QIAcuity Digital PCR System、QIAcuity Probe PCR Kit 和 QIAcuity Nanoplate 8.5K 配套使用时,不仅可提供与 qPCR 类似的端到端 dPCR 工作流程,还能够对样本中的 AAV 载体基因组拷贝进行绝对定量。
原理
AAV 裂解的一体化解决方案提供:
- AAV 裂解的标准化,具有更容易实施的标准操作程序 (SOP) 和 QC
- 病毒滴度定量的一致性
- 检测范围的宽广性,在 QIAcuity Nanoplate 8.5k 中为 2.5 拷贝/µl 至 15,000 拷贝/µl
- 稳健性,操作员和检测方案之间的变异系数 (CV) <10%
CGT Viral Vector Lysis Kit 与 QIAcuity Cell and Gene Therapy dPCR Assay 以及 QIAcuity Probe PCR Kit 相结合,可在 QIAcuity 仪器上运行单重或多重反应,从而提供完整的病毒滴度工作流程。
有关纳米微孔板中 dPCR 反应的原理说明,请参见这里。
程序
CGT Viral Vector Lysis Kit 在两个盒子中提供了用于 100 或 1000 次 AAV 载体裂解的试剂。该试剂盒适用于 AAV2、AAV5、AAV6、AAV8 和 AAV9 的裂解。裂解物经过优化,可结合 QIAcuity Probe PCR Kit 和 QIAcuity Cell and Gene Therapy dPCR Assay,用于AAV病毒滴度定量。这些检测方案能够实现单重以及多重细胞和基因治疗应用,包括病毒滴度和载体拷贝数测定。该试剂盒不包括方案(参见下面的“资源”部分)中所述的限制酶(例如 Hpall)。
应用
CGT Viral Vector Lysis Kit 用于 AAV 和腺病毒的裂解以供一系列应用使用,包括病毒载体基因组滴度和载体拷贝数测定。
辅助数据和图表
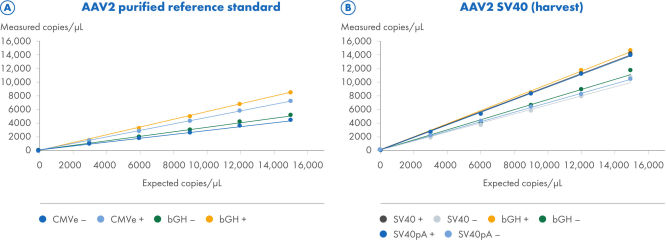
ITR digestion improves the quantification of ITR and non-ITR targets
Two AAV2 samples were processed using the CGT Viral Vector Lysis Kit and quantified using the QIAcuity Digital PCR instrument with 8.5k Nanoplates and the CGT dPCR Assays. The CGT dPCR Assays were run in triplex reactions in the FAM, HEX and Cy5 channels. The samples were serially diluted in 6 steps from 15,000 copies/µL down to 2.5 copies/µL with an R2=1.0 on 8.5k Nanoplates (A, B). Each dilution was measured in technical triplicates. Quantifications were performed with (+) and without (–) restriction digest of the ITR regions. (A) For the titration of a purified AAV2 reference standard sample, the CGT dPCR assays targeting the CMV enhancer bGH polyA regions were used. The expected copies are based on an ITR estimate determined by qPCR measurements from the reference standard supplier and not directly comparable to dPCR measurements. (B) For the titration of the AAV2-SV40 harvest sample, the CGT dPCR Assays targeting the SV40 promoter, SV40 polyA and bGH polyA regions were used.