Features
- For In Vitro Diagnostic Use
- Compatible with automated purification technologies
- Validated for various collection and storage conditions
- Unique blue cap and label identify tube for DNA testing
- 2D barcode with tube ID, lot number and expiration date
Product Details
The PAXgene Blood DNA Tube (IVD) is intended to collect, anticoagulate, stabilize, transport, and store a venous whole blood sample for preparation of human DNA for use with molecular diagnostic test methods that require DNA. The performance characteristics of this device have not been established for molecular diagnostic assays in general. Users must validate the use of a product for their specific molecular diagnostic assay. The intended use and claims have been validated with the QIAamp DSP DNA Blood Mini Kit (IVD) and the QIAsymphony DSP DNA Kit (IVD).
Performance
The performance of the PAXgene Blood DNA Tube (IVD) has been validated under several processing temperatures and time intervals relevant to variation in blood collection techniques, transport methods and storage conditions. Validation information, along with data from exposure to high temperatures, long-term storage at –20°C and freeze-thaw tests are available under the Resources tab.
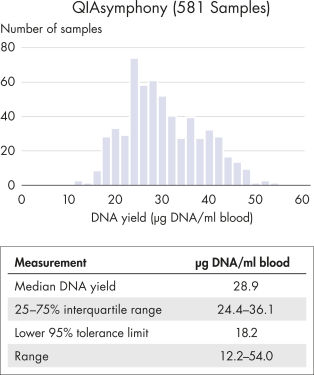
The table below summarizes DNA yields from whole blood collected in PAXgene Blood DNA Tubes (IVD) and purified using 2 different technologies. The yield of genomic DNA purified from blood samples collected in PAXgene Blood DNA Tubes (IVD) varies depending on the volume of blood processed, the white blood cell count of the specimen and the selected DNA isolation method.
| Extraction method | Number of samples | Input volume (µl) |
Output volume (µl) |
Lower 95% tolerance limit (µg DNA/ml whole blood) |
Median (µg DNA/ml whole blood) |
Mean (µg DNA/ml whole blood) |
|---|---|---|---|---|---|---|
| Magnetic beads (QIAsymphony) | 581 | 200 | 200 | 18.2 | 28.9 | 30.2 |
| Silica membrane (QIAcube) | 540 | 200 | 100 | 9.3 | 22.5 | 24.4 |
Principle
Procedure
For streamlined and standardized processing, PAXgene Blood DNA Tubes (IVD) are designed to integrate into automated workflows. For example, PAXgene Blood DNA Tubes (IVD) are compatible with DNA purification via magnetic beads in combination with QIAsymphony DSP DNA Kits (see figure DNA quality and yield from automated extraction on QIAsymphony). PAXgene Blood DNA Tubes (IVD) are also compatible with DNA purification via silica membrane in combination with QIAamp DSP DNA Blood Mini Kits (see figure DNA quality and yield from automated extraction on QIAcube).
See figures
Applications
Supporting data and figures
DNA quality and yield from automated extraction on the QIAsymphony.