REPLI-g Mitochondrial DNA Kit
对人类线粒体DNA进行高度均一的全基因组扩增
对人类线粒体DNA进行高度均一的全基因组扩增
✓ 全天候自动处理在线订单
✓ 博学专业的产品和技术支持
✓ 快速可靠的(再)订购
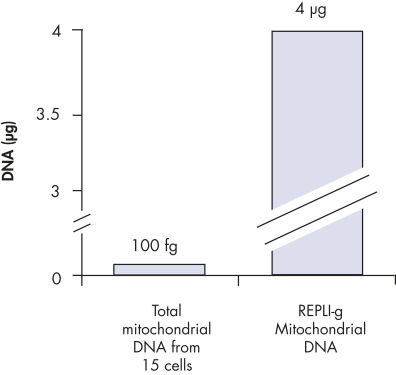
REPLI-g Mitochondrial DNA Kit可从DNA样本中选择性扩增线粒体DNA,无需预先分离线粒体DNA。该试剂盒含有DNA聚合酶、缓冲液和试剂,可利用MDA技术从少量样本中的总DNA中特异性、均一的扩增人类线粒体DNA。这一简便的操作可富集线粒体DNA,且细胞核DNA污染最小,避免进行耗时的线粒体DNA分离纯化,提高了下游分析的灵敏度。
常见的获得线粒体DNA的限制之一为需要将线粒体DNA与细胞核DNA分离,在需要提高线粒体DNA标记物分析的灵敏度时尤其如此。分离过程涉及很多耗时的步骤,有可能导致线粒体DNA的损失。REPLI-g Mitochondrial DNA Kit可在含有总DNA的样本中直接扩增获得线粒体DNA,解决了这一问题。
REPLI-g Mitochondrial DNA Kit可快速、高度均一的对线粒体基因组进行全基因组扩增。该方法基于MDA技术,采用新型独特进行性的DNA聚合酶进行等温基因组扩增。REPLI-g DNA Polymerase凭借其活跃的进行性置换反应,可扩增得到100 kb的DNA,无需预先分离DNA。与PCR扩增方法相比,这种方法能很大程度减小序列偏差和不均一位点的出现。
REPLI-g技术扩增获得的线粒体DNA可用于多种下游应用,包括:
| Features | Specifications |
|---|---|
| Amplification | Whole genomic DNA |
| Samples per run (throughput) | Mid |
| Denaturation step | Heat |
| Maximum input volume | 10 µl template DNA |
| Minimal pipetting volume needed | 1 µl |
| Reaction volume | 50 µl |
| Reaction time | ~8 hours (overnight) |
| Quality assessment | No |
| Applications | Genotyping, sequencing, RFLP |
| Starting amount of DNA | ~10 ng purified total DNA |
| Starting material | Genomic human DNA |
| Technology | Multiple Displacement Amplification (MDA) |
| Yield | ~4 µg |