✓ 全天候自动处理在线订单
✓ 博学专业的产品和技术支持
✓ 快速可靠的(再)订购
dPCR CGT Assay GFP (FAM)
Cat. No. / ID: 250236
✓ 全天候自动处理在线订单
✓ 博学专业的产品和技术支持
✓ 快速可靠的(再)订购
特点
- 广泛提供经过湿性实验室验证的十种不同 dPCR CGT 检测
- 凭借具有不同荧光基团选择的检测,可进行多重检测以证实来自一个样本的更多信息
- 与 qPCR 类似的简单快捷工作流
产品详情
腺相关病毒 (adeno-associated virus, AAV) 是在基因治疗应用中广泛使用的病毒载体。然而,病毒载体的生产和纯化需要严格的质量控制,以确保临床研究或患者治疗期间给药的安全性和可靠性。为确保基于 AAV 的基因治疗安全且有效,准确定量载体滴度和检测污染的能力至关重要。
绩效
QIAcuity Cell and Gene Therapy dPCR Assays 可广泛提供经过湿性实验室验证的十种不同 dPCR CGT 检测,具备多种荧光基团,能够以卓越的准确性、重复性和至少 4 个数量级的动态范围在多重体系构建中快速测量病毒滴度。这些检测与 QIAcuity Digital PCR System、QIAcuity Probe PCR Kit 和 QIAcuity Nanoplate 配套使用时,不仅可提供与 qPCR 类似的端到端 dPCR 工作流,还能够对样本中 AAV 载体基因组拷贝进行绝对定量。这些检测在设计时考虑到了生物制药和质量控制 (QC) 的要求。
原理
有关纳米板中 dPCR 反应的原理说明,请参见这里。
专用 CGT 检测可在 QIAcuity 上实现 AAV 定量。这些检测经过验证,并且可用于单一 和多重反应,还 可与关注的基因检测结合使用,提供:
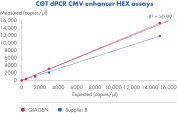
- 低至 0.3 拷贝/µl 的准确定量
- 宽动态范围内的高精度
- 在不同检测(独立于荧光基团)和操作员之间的高准确度
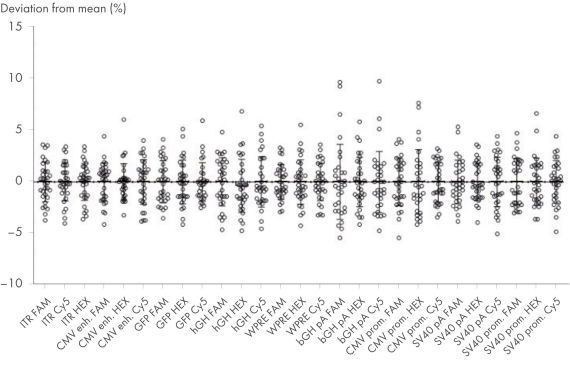
- 不依赖于荧光基团和操作员的高精度(与平均值的偏差 <10%)
- dPCR 和 qPCR 读数的兼容性
程序
QIAcuity Cell and Gene Therapy dPCR Assays 以 20x 即用型引物-探针混合物形式提供,具有多种荧光基团可供选择,并经过优化,用于与 QIAcuity Probe PCR Kit 配套使用。这些检测可实现单一和多重 CGT 应用,包括对载体滴度进行绝对定量和检测稳健性。
应用
QIAcuity Cell and Gene Therapy (CGT) dPCR Assays 适用于多种应用,包括病毒载体滴度测量、载体基因组完整性测量以及确定潜在的质粒污染(如果使用 Amp 质粒生产 AAV)。将这些 dPCR CGT Assays 纳入基因治疗开发的质量控制过程意味着可为生产出安全高效的治疗方法带来更大的确定性。
辅助数据和图表
检测方案高批间精确性
使用 AAV DNA 进行 CGT dPCR 检测。Nanoplate 8.5k内的输入量为 2500 拷贝/µl。显示了与每种检测至少 27 次重复测试测量的平均拷贝数的偏差。实验由两名操作员进行,每名操作员至少进行 13 次重复测试。