QIAquick Gel Extraction Kit – 凝胶纯化
用于从凝胶或酶促反应中凝胶提取/净化多达 10 µg 的 DNA(70 bp 至 10 kb)
用于从凝胶或酶促反应中凝胶提取/净化多达 10 µg 的 DNA(70 bp 至 10 kb)
✓ 全天候自动处理在线订单
✓ 博学专业的产品和技术支持
✓ 快速可靠的(再)订购
Cat. No. / ID: 28704
✓ 全天候自动处理在线订单
✓ 博学专业的产品和技术支持
✓ 快速可靠的(再)订购
QIAquick Gel Extraction Kit 提供离心柱、缓冲液和采样管,用于以硅胶膜为基础,从凝胶(最多 400 mg 切片)或酶促反应中纯化 DNA 片段。使用简单快速的结合-洗涤-洗脱程序和 30–50 µl 的洗脱体积,可纯化 70 bp 至 10 kb 的 DNA。集成的 pH 指示剂可轻松确定 DNA 吸附到离心柱的最佳 pH 值。QIAquick PCR & Gel Cleanup Kit 还提供用于纯化 >100 bp 的 PCR 产物和长达 10 kb 的 DNA 的缓冲液。程序可在 QIAcube Connect 上全自动完成。
为获得最佳效果,建议将本产品与 QIAvac 24 Plus 一起使用。
QIAquick Gel Extraction Kit 可去除样本中的核苷酸、酶、盐、琼脂糖、溴化乙锭和其他杂质,确保高达 80% 的 DNA 回收率(见图“ 凝胶中的高回收率”)。使用微型离心机或真空歧管,可从 1–24 个样本中纯化出 70 bp 至 10 kb 的 DNA。纯化的 DNA 可用于测序等应用(见图“ 凝胶提取后的可靠测序”)。小于 70 bp 或大于 10 kb 的 DNA 片段应使用 QIAEX II Gel Extraction System 提取。
QIAquick PCR 纯化程序可去除 DNA 样本中的引物、核苷酸、酶、矿物油、盐和其他杂质(见图“ PCR 后完全去除引物”)。使用微型离心机或真空歧管,可纯化出 100 bp 至 10 kb 的 DNA。
QIAquick Kit 包含一个硅胶膜组件,用于在高盐缓冲液中结合 DNA,然后用低盐缓冲液或水进行洗脱。纯化程序可去除 DNA 样本中的引物、核苷酸、酶、矿物油、盐、琼脂糖、溴化乙锭和其他杂质(见图“ 凝胶中的高回收率”)。硅胶膜技术消除了树脂松散和稀浆带来的问题和不便。专用结合缓冲液针对特定应用进行了优化,可促进特定大小范围内 DNA 分子的选择性吸附。
为了更快、更方便地进行样本处理和分析,我们提供了凝胶加载染料。GelPilot Loading Dye 包含三种跟踪染料(二甲苯胍青、溴酚蓝和橙黄 G),便于优化琼脂糖凝胶的运行时间,防止较小的 DNA 片段迁移过远(见图“ GelPilot Loading Dye”)。
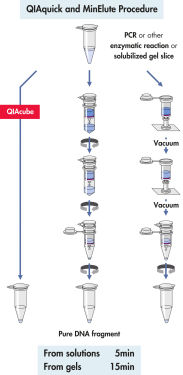
QIAquick 系统使用简单的结合-洗涤-洗脱程序(见流程图“ QIAquick 和 MinElute 程序”)。将凝胶切片溶解在含有 pH 指示剂的缓冲液中,以便确定 DNA 结合的最佳 pH 值,然后将混合物加到 QIAquick 离心柱上(见图“ pH 指示基团染料”)。核酸在缓冲液提供的高盐条件下吸附到硅胶膜上。杂质被洗去,用少量低盐缓冲液或水洗脱出的纯 DNA 可直接用于所有后续应用。
QIAquick 离心柱的设计提供了两种方便的处理选项。离心柱可安装到传统的台式微型离心机或任何带鲁尔接头的真空歧管上,如带有 QIAvac Luer Adapter 的 QIAvac 24 Plus。QIAquick Gel Extraction Kit 以及其他基于 QIAGEN 离心柱的试剂盒均可在 QIAcube Connect 上实现全自动操作,从而提高生产效率和结果的规范化(见图“离心柱处理选项 A、 B、 C、 D”和“ QIAcube Connect”)。
用 QIAquick 系统纯化的 DNA 片段可直接用于所有应用包括测序、微阵列分析、连接和转化、限制性酶切、标记、PCR 和体外转录。
| Features | Specifications |
|---|---|
| Binding capacity | 10 µg |
| Format | 试管 |
| Fragment size | 70 bp – 10 kb |
| Recovery: oligonucleotides dsDNA | 回收率:dsDNA 片段 |
| Processing | 手动 |
| Removal <10mers 17–40mers dye terminator proteins | 去除 <10mer |
| Elution volume | 30-50 µl |
| Technology | 硅胶膜技术 |
| Sample type: applications | DNA:PCR 反应 |