Products
QIAsymphony DSP DNA Kits可用于体外诊断。

QIAsymphony DSP DNA Mini Kit (192) 旨在用于体外诊断用途。
特点
- 适用于多种类型样本的DNA纯化
- 方便易用的创新性预装试剂条
- 标准化操作流程带来的结果高度可重复性
- 附带的条形码扫描功能可用于样本和试剂的全程追踪
产品详情
QIAsymphony DSP DNA Kits系列试剂盒可配套QIAsymphony SP仪器用于从人类全血、白膜层、组织、FFPE组织、培养的细胞及细菌等样本中进行DNA的自动化提取,也可用于体外诊断实验所需的人类全血中病毒DNA的自动化提取。该类试剂盒可分为小量和中量提取两种规格,适用于体积从200 µl到1000 µl的起始样本。对于组织、FFPE组织、培养的细胞及细菌样本,手工操作的样本预处理过程是必要的。经优化的一系列操作步骤可帮助从样本中获得较高产量的纯化DNA,灵活的洗脱体积为下游多种应用所需的不同DNA浓度优化提供了可能。创新的预装试剂条则能够最大程度的减少了实验设置和手工操作的时间。附带的条形码扫描功能可用于相关试剂的全程追踪。
原理
QIAsymphony DSP DNA Kits系列试剂盒可用于人类全血、白膜层、组织、FFPE组织、培养的细胞及细菌等样本中DNA的提取,也可用于人类全血中病毒DNA的提取。
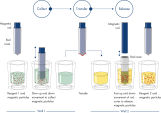
QIAsymphony DSP DNA Kits系列试剂盒将DNA提取过程中硅胶膜法所具有的快速、高效优势同磁珠法所具有的操作便捷性进行了很好的结合(参见 QIAsymphony SP原理示意图)。创新的即用型试剂预装条里已预装了纯化步骤所需的全部试剂,并且会由QIAsymphony SP仪器进行自动开封,最大程度地减少了环境污染的风险(参见 预装封闭的试剂条)。工作台可进行快速设置,可节省宝贵的时间。实验时只需简单的将来自不同或相同试剂盒的最多2个试剂条放置于试剂抽屉中即可,在一次仪器运行过程中对于最多96个样本可同时运行多种不同的纯化步骤。相关试剂会根据样本量进行配套使用,不会造成试剂浪费而达到最大程度的成本控制。
QIAsymphony DSP DNA Kits系列试剂盒将DNA提取过程中硅胶膜法所具有的快速、高效优势同磁珠法所具有的操作便捷性进行了很好的结合(参见 QIAsymphony SP原理示意图)。创新的即用型试剂预装条里已预装了纯化步骤所需的全部试剂,并且会由QIAsymphony SP仪器进行自动开封,最大程度地减少了环境污染的风险(参见 预装封闭的试剂条)。工作台可进行快速设置,可节省宝贵的时间。实验时只需简单的将来自不同或相同试剂盒的最多2个试剂条放置于试剂抽屉中即可,在一次仪器运行过程中对于最多96个样本可同时运行多种不同的纯化步骤。相关试剂会根据样本量进行配套使用,不会造成试剂浪费而达到最大程度的成本控制。
查看图表
程序
该纯化步骤是为确保对潜在可感染样本进行处理时的安全性和可重复性而设计的,分为4步:裂解、吸附、洗涤以及洗脱(参见QIAsymphony DSP DNA提取步骤)。经优化的实验操作步骤在获取高质量核酸的基础上还能高效的去除蛋白、核酸酶以及其他杂质。经QIAsymphony DSP DNA Kits系列试剂盒纯化得到的核酸可直接适用于下游扩增或是其他酶反应的诸多应用。该试剂盒可灵活选择不同的洗脱体积。
查看图表
应用
QIAsymphony DSP DNA Kits系列试剂盒纯化得到的高品质核酸可直接适用于下游的多种应用,如测序或是诸如实时定量PCR、RT-PCR等较为灵敏的检测实验。
辅助数据和图表
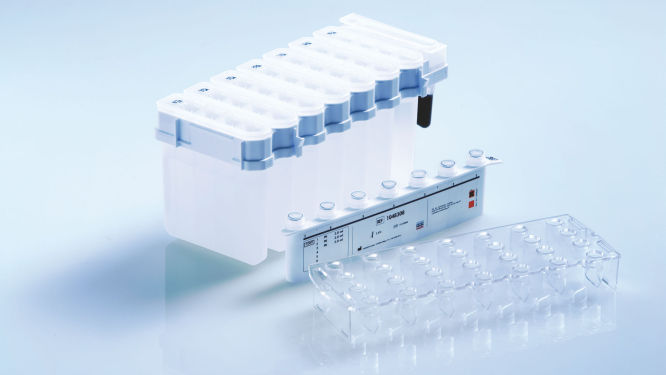
Prefilled, sealed reagent cartridges.
Prefilled, sealed reagent cartridges.
资源
Application/Protocol Documents for IVD Use (14)
安全数据表 (1)
性能数据 (2)
耗材文档 (2)
Technical Information and Important Notes (1)
Application/Protocol Documents for User-Validated Use (1)
试剂盒操作手册 (2)
Safety Data Sheets (1)
Certificates of Analysis (1)
FAQ
What sample types can be processed using the tissue protocols and the QIAsymphony DSP DNA Mini Kit?
How much starting material can be used with the QIAsymphony DSP DNA Mini Kit on the QIAsymphony SP?
Do tissue samples require pretreatment before loading onto the QIAsymphony SP for DNA extraction?
What sample volumes can be used with the tissue HC and LC protocols on the QIAsymphony SP?
What are the recommended lysis times for tissue samples on the QIAsymphony SP?
What sample types should be processed with the low content (LC) and high content (HC) protocols for DNA extraction on the QIAsymphony SP?
Will mitochondrial DNA also be isolated using the DNA tissue protocols and QIAsymphony DSP DNA Mini Kit on the QIAsymphony SP?