QIAamp DSP DNA Blood Mini Kit
从人类全血中纯化基因组DNA
从人类全血中纯化基因组DNA
✓ 全天候自动处理在线订单
✓ 博学专业的产品和技术支持
✓ 快速可靠的(再)订购
Cat. No. / ID: 61104
✓ 全天候自动处理在线订单
✓ 博学专业的产品和技术支持
✓ 快速可靠的(再)订购
QIAamp DSP DNA Blood Mini Kit采用硅胶模技术纯化DNA,适用于进行血液DNA纯化的实验室。
使用QIAamp DSP DNA Blood Mini Kit纯化的基因组DNA可即用于基于酶扩增或其他修饰的各种下游应用,如PCR。
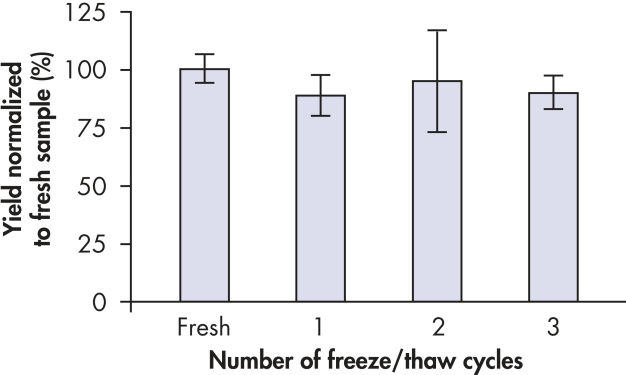
全血样品使用装有抗凝剂(EDTA或柠檬酸盐)的不同类型的管子收集,包括:BD Vacutainer、Monovette和Vacuette管(参见下表)。血液样品可反复冻融至少3次,仍可用于DNA纯化(参见" DNA from frozen and thawed blood")。
| 管子 | 生产商 | 编号 | 体积 | 200 µl的平均产量 |
|---|---|---|---|---|
| BD Vacutainer 9NC | BD | 366007 | 9 ml | 6.4 µg |
| BD Vacutainer K3E | BD | 368457 | 10 ml | 6.6 µg |
| BD Vacutainer K2E | BD | 367864 | 6 ml | 6.4 µg |
| S-Monovette EDTA | Sarstedt | 02.1066.001 | 9 ml | 6.5 µg |
| S-Monovette CPDA1 | Sarstedt | 01.1610.001 | 8.5 ml | 6.3 µg |
| Vacuette K3E | Greiner Bio-One | 455036 | 9 ml | 6.5 µg |
| Vacuette 9NC | Greiner Bio-One | 454382 | 2 ml | 6.3 µg |
QIAamp DSP DNA Blood Mini Kit采用成熟的QIAamp技术纯化基因组DNA。QIAamp硅胶膜特异性结合裂解样品中的DNA,其他裂解物通过离心或真空操作去除。经过有效地洗涤去除污染物,然后洗脱DNA,洗脱体积为50–200 µl。
有两个可供选择的流程用于从血液中纯化基因组DNA,使用离心机或使用真空抽滤装置和离心机(参见" Procedure")。
含有EDTA或柠檬酸盐的血液样品(200 µl)用含QIAGEN Protease的裂解缓冲液56℃裂解10分钟。乙醇加入到裂解物中,使DNA特异性结合到QIAamp硅胶膜上。裂解物上样到QIAamp Mini离心柱,离心或真空处理。DNA特异性结合到QIAamp硅胶膜上,污染物流走。结合的DNA使用两种不同的缓冲液有效洗涤,离心干缩QIAamp硅胶膜。洗脱缓冲液(50–200 µl)上样到QIAamp硅胶膜,1分钟后离心洗脱纯的DNA。
QIAamp DSP DNA Blood Mini Kit使用经验证的QIAamp技术从新鲜或冷冻的全血中纯化DNA,其中血液是使用柠檬酸盐或EDTA处理过的。
EDTA-treated blood samples were frozen and thawed up to 3 times. DNA was purified at the end of each freeze-thaw cycle. DNA was purified from 200 µl samples using the QIAamp DSP DNA Blood Mini Kit and eluted in 200 µl elution buffer. DNA yields are normalized to the yield from fresh sample (100%). Each bar represents the results from 32 replicates (mean ± standard deviation).
| Features | Specifications |
|---|---|
| Applications | PCR, qPCR, real-time RT-PCR, microarray |
| Main sample type | Whole blood |
| Elution volume | 50–200 µl |
| Purification of total RNA, miRNA, poly A+ mRNA, DNA or protein | Genomic DNA |
| CE/FDA/IVD compatible | CE/IVD |
| Format | Spin columns |
| Sample amount | 200 µl |
| Processing | Manual (centrifugation or vacuum) |
| Technology | Silica technology |