Products

Cell and Gene Therapy Viral Vector Lysis Kit (100 rxn)
Cat. No. / ID: 250272

Cell and Gene Therapy Viral Vector Lysis Kit (1000 rxn)
Cat. No. / ID: 250273
特点
- 使用一个 AAV 裂解试剂盒和一种方案使当前工作流程的标准化和质量控制 (Quality Control, QC)成为可能
- 对多种血清型(如 AAV 血清型)的病毒滴度进行一致且可重复的测定
- 与 QIAcuity Cell and Gene Therapy (CGT) dPCR Assay 相结合的完整工作流程,可在 QIAcuity Digital PCR System 上实现 AAV 滴度的准确测定
产品详情
腺相关病毒是在基因治疗应用领域广泛使用的一种病毒载体。然而,病毒载体的生产和纯化过程需要精密的质量控制,以确保临床研究或患者治疗期间给药的安全性和可靠性。准确定量载体滴度以及确定污染的能力是安全且有效的基于 AAV 的基因治疗的关键。
随着 CGT Viral Vector Lysis Kit(包含用于 100 或 1000 次反应的试剂)的推出,我们现在拥有了一个经过优化的和标准化的病毒载体裂解工作流程,可以准确且精密地测定病毒滴度。该试剂盒也可用于腺病毒裂解。
绩效
我们提供了从进行 AAV 裂解到在细胞裂解液中定量病毒滴度的完整工作流程。CGT Viral Vector Lysis Kit 及其改进的配方可对最终滴度进行一致、稳健且准确的测定。它与 QIAcuity Cell and Gene Therapy dPCR Assay、QIAcuity Digital PCR System、QIAcuity Probe PCR Kit 和 QIAcuity Nanoplate 8.5K 配套使用时,不仅可提供与 qPCR 类似的端到端 dPCR 工作流程,还能够对样本中的 AAV 载体基因组拷贝进行绝对定量。
原理
AAV 裂解的一体化解决方案提供:
- AAV 裂解的标准化,具有更容易实施的标准操作程序 (SOP) 和 QC
- 病毒滴度定量的一致性
- 检测范围的宽广性,在 QIAcuity Nanoplate 8.5k 中为 2.5 拷贝/µl 至 15,000 拷贝/µl
- 稳健性,操作员和检测方案之间的变异系数 (CV) <10%
CGT Viral Vector Lysis Kit 与 QIAcuity Cell and Gene Therapy dPCR Assay 以及 QIAcuity Probe PCR Kit 相结合,可在 QIAcuity 仪器上运行单重或多重反应,从而提供完整的病毒滴度工作流程。
程序
CGT Viral Vector Lysis Kit 在两个盒子中提供了用于 100 或 1000 次 AAV 载体裂解的试剂。该试剂盒适用于 AAV2、AAV5、AAV6、AAV8 和 AAV9 的裂解。裂解物经过优化,可结合 QIAcuity Probe PCR Kit 和 QIAcuity Cell and Gene Therapy dPCR Assay,用于 AAV 病毒滴度定量。这些检测方案能够实现单重以及多重细胞和基因治疗应用,包括病毒滴度和载体拷贝数测定。该试剂盒不包括方案(参见下面的“资源”部分)中所述的限制酶(例如 Hpall)。
应用
CGT Viral Vector Lysis Kit 用于 AAV 和腺病毒的裂解以供一系列应用使用,包括病毒载体基因组滴度和载体拷贝数测定。
辅助数据和图表
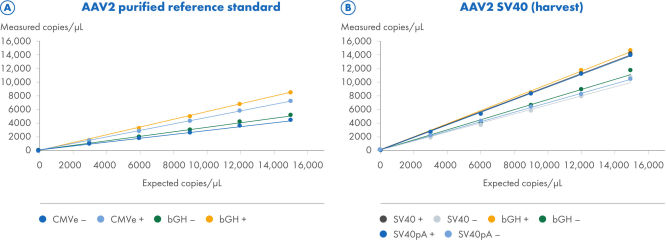
ITR 消化改善了 ITR 和非 ITR 靶标的定量
使用 CGT Viral Vector Lysis Kit 处理了两个 AAV2 样本,并使用 QIAcuity Digital PCR 仪器配合 8.5k 纳米板和 CGT dPCR 检测进行了定量。CGT dPCR 检测在 FAM、HEX 和 Cy5 通道中以三重反应方式运行。在 8.5k 纳米板(A、B)上分 6 个步骤将样本从 15,000 个拷贝/µL 连续稀释至 2.5 个拷贝/µL,R2=1.0。每次稀释都使用三个技术复制本进行测量。在使用 (+) 和不用 (–) ITR 区域限性消化的情况下进行了定量。(A) 为了滴定纯化的 AAV2 参考标准样本,使用了靶向 CMV 增强子 bGH polyA 区域的 CGT dPCR 检测。预期拷贝数基于通过参比标准品供应商的 qPCR 测量值确定的 ITR 估计值,而非与 dPCR 测量值直接比较。(B) 为了滴定 AAV2-SV40 采集样本,使用了靶向 SV40 启动子、SV40 polyA 和 bGH polyA 区域的 CGT dPCR 检测。