EndoFree Plasmid Kit
纯化得到至多10 mg的无内毒素高转染级纯质粒或柯斯质粒DNA
纯化得到至多10 mg的无内毒素高转染级纯质粒或柯斯质粒DNA
✓ 全天候自动处理在线订单
✓ 博学专业的产品和技术支持
✓ 快速可靠的(再)订购
Cat. No. / ID: 12362
✓ 全天候自动处理在线订单
✓ 博学专业的产品和技术支持
✓ 快速可靠的(再)订购
EndoFree Plasmid Kits基于阴离子交换技术,可快速制备不含内毒素的质粒DNA。QIAfilter Cartridges过滤快速澄清裂解液。制备的DNA纯度超过两次CsCl梯度离心法所得DNA的纯度,适合超转染级纯的应用。EndoFree Plasmid Buffer Set可用于制备10次mega规模或5次giga规模转染级纯质粒或科斯质粒DNA制备。
EndoFree Plasmid Kit将高效的内毒素去除步骤整合到质粒纯化流程中,不需要额外步骤,也不需用亲和柱去除脂多糖。通过QIAfilter Mega-Giga Cartridge过滤细菌裂解液,并使用重力流QIAGEN-tip阴离子交换柱纯化质粒DNA。产量可达10 mg(Giga)、2.5 mg(Mega)、500 μg(Maxi)DNA。纯化的DNA无内毒素(<0.1 EU/µg DNA)。
选用EndoFree Plasmid Mega Kit而非EndoFree Plasmid Giga Kit纯化低拷贝质粒和科斯质粒,将会得到更好的效果,因为后者需要较大的培养体积,而QIAfilter Mega-Giga Cartridge的容量有限。
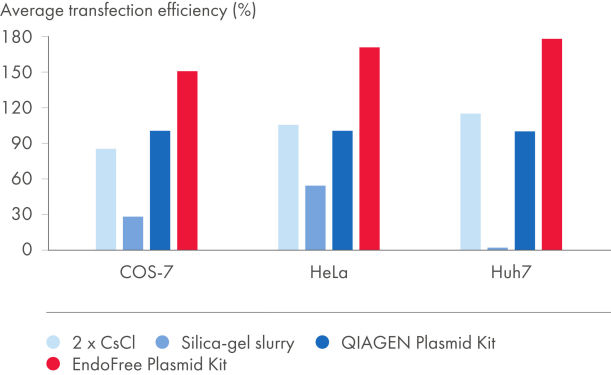
EndoFree Plasmid Kits去除细菌在裂解过程中释放的内毒素,内毒素会影响原代细胞和敏感培养细胞的DNA转染。从EndoFree Plasmid Kits得到的无内毒素DNA高度适用于可重复且结果可靠的转染(参见"Plasmid purification method versus transfection efficiency"和"Plasmid purity versus transfection efficiency"及表"Endotoxin levels in plasmid preparations"和"EndoFree DNA yields high transfection efficiencies with primary cells")。QIAGEN超纯无内毒素DNA也适用于基因治疗研究和其他敏感应用。
| 质粒制备方法 | 内毒素(EU†/µg DNA) | 平均转染效率‡ |
|---|---|---|
| EndoFree Plasmid Kit | 0.1 | 154% |
| QIAGEN Plasmid Kit | 9.3 | 100% |
| 2x CsCl | 2.6 | 99% |
| Silica-gel slurry | 1230.0 | 24% |
| DNA纯化方法 | 转染细胞比例 |
|---|---|
| EndoFree Plasmid Kit | 21.0% ± 0.93 |
| QIAGEN Plasmid Kit | 8.1% ± 0.57 |
| Silica-gel slurry | 5.2% ± 0.74 |
纯化的质粒DNA内毒素污染水平取决于所用的纯化方法(参见"Endotoxin levels in plasmid preparations")。硅胶技术纯化的DNA具有非常高的内毒素水平。QIAGEN、QIAfilter、HiSpeed Plasmid Kits和两次CsCl超速离心可获得相对低水平内毒素的纯DNA。EndoFree Plasmid Buffer Set包含完整的内毒素去除步骤,获得<0.1 EU/µg的质粒DNA。
QIAfilter、HiSpeed和EndoFree Plasmid Kits提供的QIAfilter Cartridges是特殊设计的过滤装置,用于代替细菌细胞碱裂解后的离心步骤。QIAfilter Cartridges可完全去除SDS沉淀,清除细菌裂解液,相比离心可大量节约时间,减少1小时的质粒纯化时间。QIAfilter Mega-Giga Cartridges采用内部真空方式轻松高效的清除大量细菌裂解液(注意:该试剂盒不包含瓶子)。
QIAGEN-tips中独特的阴离子交换树脂(不提供EndoFree Plasmid Buffer Set)专为核酸纯化而开发。它卓越的分离性能使得DNA纯度与连续两次CsCl梯度离心获得的DNA纯度相当。由重力流驱动并持续运转的预装QIAGEN-tips,最大限度减少手动制备质粒所需的时间。整个QIAGEN质粒纯化系统不使用有毒物质,如苯酚,氯仿,溴化乙锭和CsCl,最大限度地减少对用户和环境的危害。内毒素,又称脂多糖或LPS,是大肠杆菌等革兰氏阴性菌细胞膜的组成部分,如大肠杆菌(参见"Bacterial cell wall")。内毒素在质粒裂解纯化过程中释放出来,并显著降低内毒素敏感细胞株的转染效率。此外,内毒素与DNA竞争“自由”转染试剂,影响转染实验中质粒DNA的摄取。内毒素也诱导巨噬细胞和B细胞等免疫细胞发生非特异性免疫反应,从而导致转染结果的误读。这些反应包括诱导的蛋白质和脂类,如IL-1和前列腺素等的合成。总体而言,内毒素在转染实验中是不可控变量,影响结果的可重复性,使它们难以对比、解读。在基因治疗研究中,内毒素可造成内毒素休克综合症和激活补体级联,干扰研究。
在碱性条件下,细菌细胞裂解,使用QIAfilter Mega-Giga Cartridge清除粗裂解物。将Endotoxin Removal Buffer加入到过滤后的裂解液中。冰浴后,将纯净的裂解物上样到阴离子交换柱上,在适当的低盐和pH条件下,质粒DNA选择性结合。用中盐缓冲液清除RNA、蛋白质、代谢产物和其他低分子量杂质,超纯质粒DNA被高盐缓冲液洗脱(参见"QIAGEN Plasmid Kit procedures")。最后DNA由异丙醇脱盐、沉淀并且离心回收。
使用EndoFree Plasmid Kits纯化的DNA适合多种敏感应用,包括: