QuantiTect Probe PCR Kit (200)
Cat. No. / ID: 204343
特点
- 高度灵敏的低拷贝靶标检测
- 使用序列特异性探针或 SYBR Green 的 qPCR
- 对跨越多个数量级的模板进行精确定量
- 无需优化反应和循环反应条件
- 在一管反应中检测多达 5 个靶标
产品详情
QuantiTect PCR Kit(qPCR 试剂盒)可使用序列特异性探针或 SYBR Green I 检测,通过 Real-time PCR 和两步法 RT-PCR 对 gDNA 和 cDNA 靶标进行灵敏定量。这些 Real-time PCR 试剂盒还能通过多重 Real-time PCR 或两步法 RT-PCR,在一管反应中可靠地定量多达 5 个 gDNA 或 cDNA 靶标。即用型预混液中热启动和独特 PCR 缓冲液系统相结合,可确保在任何实时定量 PCR 仪上无需优化便可进行高灵敏度的 qPCR。dNTP 混合物包括 dUTP,可选择用 UNG 处理。为方便起见,QuantiTect PCR Kit 中的预混液可存放在 2–8°C 环境下。
有两种试剂盒形式可用于使用序列特异性探针的多重 PCR:适用于需要 ROX 染料进行荧光归一化处理的定量 PCR 仪的 QuantiTect Multiplex PCR Kit;适用于所有其他定量 PCR 仪的 QuantiTect Multiplex PCR NoROX Kit。
绩效
与其他聚合酶相比,QuantiTect 定量 PCR 试剂盒中包括的 HotStarTaq DNA Polymerase 能提供最严格的热启动,从而提高 PCR 反应的特异性。
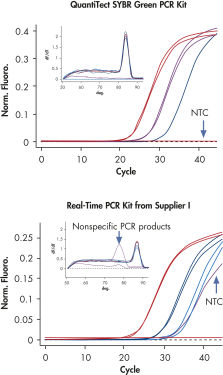
QuantiTect SYBR Green PCR Kit 可在宽线性范围内进行特异性定量(请参阅图“ 宽线性范围内的特异性定量”)。与 QuantiTect Reverse Transcription Kit 和 QuantiTect Primer Assay 结合使用时,QuantiTect SYBR Green PCR Kit 可提供灵敏可靠的结果(请参阅图“ 具有特异性且灵敏的定量”)。
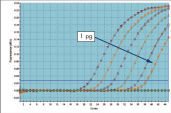
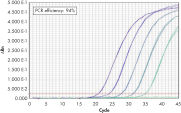
QuantiTect Probe PCR Kit 与 QuantiTect Reverse Transcription Kit 结合使用,可获得灵敏可靠的结果(请参阅图“ 灵敏度和效率高,动态范围广”)。独特的 PCR 缓冲液成分使 QuantiTect Probe RT-PCR Kit 能对低拷贝 DNA 靶标进行灵敏定量,并能在宽线性范围内准确定量(请参阅图“ Real-time PCR 动态范围广”)。
QuantiTect Multiplex PCR Master Mix 可确保多重反应中 PCR 产物的扩增效率和灵敏度与相应单重扩增反应中的 PCR 产物相同(请参阅表“三重和单重 PCR” 的阈值循环 (CΤ) 值相当和图 “ 4 重和单重 PCR 的结果相当”)。
| 检测 t(8;14) 易位序列(20 个拷贝) | 检测 GAPDH cDNA 序列(106 个拷贝) | 检测 NFKB cDNA 序列(拷贝数见第一列) | |
|---|---|---|---|
| 具有 105 个 NFKB 拷贝的三重 PCR | 34.31 | 20.37 | 21.92 |
| 对应的单重 PCR | 34.07 | 20.54 | 21.83 |
| 具有 104 个 NFKB 拷贝的三重 PCR | 34.61 | 20.62 | 25.03 |
| 对应的单重 PCR | 34.00 | 20.46 | 25.19 |
| 具有 103 个 NFKB 拷贝的三重 PCR | 35.17 | 19.94 | 28.38 |
| 对应的单重 PCR | 34.43 | 20.50 | 28.65 |
即使同一反应中参考基因的拷贝数多达 106 倍,这些试剂盒也能检测到低至 10 个拷贝的靶标基因(请参阅图“ 在存在过量参考基因的情况下检测 10 个拷贝的靶标基因和相应的表格“高低丰度靶标的成功定量三重 qPCR”)。
| 检测 CSBG | 检测 GAPDH | 检测 HSP | |
| 模板混合物 1 | 1400 个拷贝 | 106 个拷贝 | 2 x 104 个拷贝 |
|---|---|---|---|
| 三重 PCR 的 CT 值 | 27.73 | 18.69 | 23.59 |
| 单重 PCR 的 CT 值 | 27.08 | 18.89 | 23.52 |
| 模板混合物 2 | 140 个拷贝 | 106 个拷贝 | 2 x 103 个拷贝 |
| 三重 PCR 的 CT 值 | 31.11 | 19.00 | 27.05 |
| 单重 PCR 的 CT 值 | 30.66 | 18.61 | 26.97 |
| 模板混合物 3 | 14 个拷贝 | 106 个拷贝 | 2 x 102 个拷贝 |
| 三重 PCR 的 CT 值 | 34.74 | 18.98 | 30.71 |
| 单重 PCR 的 CT 值 | 33.84 | 19.01 | 30.38 |
查看图表
原理
QuantiTect SYBR Green PCR Kit 含有经过优化的即用型预混液,可使用 SYBR Green I 对 cDNA 靶标进行高特异性和灵敏度的实时定量(请参阅表 “2x QuantiTect SYBR Green PCR Kit 的组分”)。预混液中的荧光染料 SYBR Green I 可用于分析多种靶标,而无需合成靶标特异性标记探针。PCR 缓冲液中 K+ 和 NH4+ 离子的平衡组合可促进特异性引物退火,实现高 PCR 特异性和灵敏度(请参阅图“特异性引物退火”)。此外,HotStarTaq DNA Polymerase 可提供严格的热启动,防止形成非特异性产物。
| 组分 | 特点 | 优势 |
| HotStarTaq DNA Polymerase | 在 95ºC 下 15 分钟活化 | 在室温下配制 qPCR 反应 |
| QuantiTect SYBR Green PCR Buffer | NH4+ 和 K+ 离子的平衡组合 | 特异性引物退火确保可靠的 PCR 结果 |
| dNTP 混合物 | 包括 dUTP,可部分取代 dTTP,并可进行可选的 UNG 反应处理 | 通过可选的 UNG 处理消除 PCR 产物的残留污染 |
| SYBR Green I 染料 | 结合双链 DNA 后产生强荧光信号 | 高灵敏度定量 |
| ROX 染料 | 用于对 Applied Biosystems 并可选择在 Agilent 仪器上对荧光信号进行归一化处理 | 可在需要 ROX 染料的定量 PCR 仪上精确定量。不会干扰其他实时定量 PCR 仪上的反应 |
QuantiTect Probe PCR Kit 含有经过优化的即用型预混液,可使用序列特异性探针对 gDNA 和 cDNA 靶标进行高特异性和灵敏度的实时定量(请参阅表 “2x QuantiTect Probe PCR Kit 的组分”)。这些试剂盒设计用于所有类型的序列特异性探针,包括水解探针检测(例如 TaqMan® 和其他双标记探针)、FRET 探针及分子信标。QuantiTect Probe PCR Kit 含有独特的 PCR 缓冲液,其中包含 K+ 和 NH4+ 离子的平衡组合,可促进特异性引物退火,从而实现高 PCR 特异性和灵敏度(请参阅图“特异性引物退火”)。此外,HotStarTaq DNA Polymerase 可提供严格的热启动,防止形成非特异性产物。
| 组分 | 特点 | 优势 |
| HotStarTaq DNA Polymerase | 在 95ºC 下 15 分钟活化 | 在室温下配制 qPCR 反应 |
| QuantiTect Probe PCR Buffer | NH4+ 和 K+ 离子的平衡组合 | 特异性引物退火确保可靠的 PCR 结果 |
| dNTP 混合物 | 包括 dUTP,可部分取代 dTTP,并可进行可选的 UNG 反应处理 | 通过可选的 UNG 处理消除 PCR 产物的残留污染 |
| ROX 染料 | 用于对 Applied Biosystems 并可选择在 Agilent 仪器上对荧光信号进行归一化处理 | 可在需要 ROX 染料的定量 PCR 仪上精确定量。不会干扰其他实时定量 PCR 仪上的反应 |
QuantiTect Multiplex PCR Kit 使多重、两步法 RT-PCR 首次尝试就能成功(请参阅流程图“ QIAGEN 多重试剂盒”)。经优化的预混液可确保多重反应中 PCR 产物的扩增效率和灵敏度与相应单重扩增反应中的 PCR 产物相同。使用该试剂盒可检测到低至 10 个拷贝的靶标基因。
在同一而非独立反应中扩增对照和靶标基因可尽量减少操作错误,从而提高基因定量的可靠性。QuantiTect Multiplex PCR Master Mix 包含 K+ 和 NH4+ 离子的平衡组合以及独特的 Synthetic Factor MP,它们能共同促进引物和探针稳定且高效地退火到核酸模板,从而提高 PCR 的效率(请参阅表“2x QuantiTect Multiplex PCR Kit 的组分”)。此外,HotStarTaq DNA Polymerase 可提供严格的热启动,防止形成非特异性产物。
QuantiTect PCR Kit 中的预混液还含有 dUTP,可在开始 PCR 之前用尿嘧啶-N-糖基化酶 (Uracil-N-Glycosylase, UNG) 进行预处理,确保任何具有污染性的 PCR 产物都不会影响后续的 PCR 反应。
| 组分 | 特点 | 优势 |
| HotStarTaq DNA Polymerase | 在 95ºC 下 15 分钟活化 | 在室温下配制 qPCR 反应 |
| QuantiTect Multiplex PCR Buffer | NH4+ 和 K+ 离子的平衡组合 | 特异性引物退火确保可靠的 PCR 结果 |
| Synthetic Factor MP | 可在一管反应中对多达 4 个基因进行可靠的多重分析 | |
| dNTP 混合物 | 包括 dUTP,可部分取代 dTTP,并可进行可选的 UNG 反应处理 | 通过可选的 UNG 处理消除 PCR 产物的残留污染 |
| ROX 染料* | 在 Applied Biosystems 并可选择在 Agilent 仪器上对荧光信号进行归一化处理 | 可在需要 ROX 染料的定量 PCR 仪上精确定量。不会干扰其他实时定量 PCR 仪上的反应 |
查看图表
程序
如有需要,可用尿嘧啶-N-糖基化酶 (Uracil-N-Glycosylase, UNG) 对反应进行预处理,以消除先前反应中 PCR 产物的残留污染。为获得最佳的实时两步法 RT-PCR 结果,我们建议使用 QuantiTect Reverse Transcription Kit 合成 cDNA。该试剂盒能在短短 20 分钟内快速合成 cDNA,并集成了去除基因组 DNA 污染功能。
QuantiTect SYBR Green 和 Probe PCR Kit 无需进行可能既繁琐又耗时的反应条件优化。只需在即用型 PCR 预混液中加入引物和 DNA 模板,便可启动反应。按照手册中的方案,在任何实时定量 PCR 仪上获得快速可靠的结果。
将 QuantiTect SYBR Green PCR Kit 与 QuantiTect Primer Assay 结合使用,可确保获得高特异性的基因表达分析结果。这些引物是经过生物信息验证的全基因组引物集,可用于检测人、小鼠、大鼠和许多其他物种的转录物。您可以通过 GeneGlobe 方便地在线订购 QuantiTect Primer Assay。
QuantiTect Multiplex PCR Kit 包含即用型预混液,无需优化反应和循环条件。该手册包含一个可用于所有实时定量 PCR 仪的单一方案,还列出了推荐使用的染料。
试剂盒提供时预混液中可包含或不含 ROX 参比荧光染料,因此几乎可在任何实时定量 PCR 仪上使用(请参阅表“选择合适的 QuantiTect Multiplex PCR Kit”)。由于 ROX 浓度经过优化,即使是低拷贝数也能通过自动数据分析检测出来。
| ROX 染料 | 试剂盒 | 兼容的定量 PCR 仪 |
| 在预混液中提供 | QuantiTect Multiplex PCR Kit | Applied Biosystems 的循环仪 |
| 预混液中不存在 | QuantiTect Multiplex PCR NoROX Kit | Rotor-Gene 定量 PCR 仪,以及来自 Bio-Rad、Cepheid、Eppendorf、Roche、Agilent 及其他供应商的定量 PCR 仪 |
应用
QuantiTect PCR Kit 可用于在任何实时定量 PCR 仪上进行 cDNA 基因表达分析或 gDNA 定量。这包括来自 Applied Biosystems、Bio-Rad、Cepheid、Eppendorf、Roche 和 Agilent 的仪器。对于 Rotor-Gene Q 和其他 Rotor-Gene 定量 PCR 仪,我们建议使用 Rotor-Gene SYBR Green PCR Kit、Rotor-Gene Probe PCR Kit 或 Rotor-Gene Multiplex PCR Kit,它们是专为在这些仪器上进行快速循环反应而开发的。
| 特点 | QuantiTect SYBR Green PCR Kit | QuantiTect Probe PCR Kit | QuantiTect Multiplex PCR Kit |
| 应用 | 基因组 DNA 或 cDNA 靶标的实时定量 | 基因组 DNA 或 cDNA 靶标的实时定量 | 基因组 DNA 或 cDNA 靶标的实时定量 |
| 反应类型 | PCR 和两步法 RT-PCR | PCR 和两步法 RT-PCR | 多重 PCR 和多重两步法 RT-PCR |
| 实时或终点 | 实时 | 实时 | 实时 |
| 样本/靶标类型 | DNA、cDNA | DNA、cDNA | DNA、cDNA |
| 单重或多重 | 单重 | 单重 | 多重 |
| SYBR Green I 或序列特异性探针 | SYBR Green I | 序列特异性探针 | 序列特异性探针 |
| 热循环仪 | 所有实时定量 PCR 仪(例如 LightCycler、Rotor-Gene、ABI) | 大多数实时定量 PCR 仪(例如 LightCycler、Rotor-Gene、ABI) | 大多数实时定量 PCR 仪(例如 LightCycler、Rotor-Gene、ABI) |
| 有或无 ROX | 有 ROX | 有 ROX | 有或无 ROX |
辅助数据和图表
宽线性范围内的特异性定量。