✓ 全天候自动处理在线订单
✓ 博学专业的产品和技术支持
✓ 快速可靠的(再)订购
Effectene Transfection Reagent (1 ml)
Cat. No. / ID: 301425
✓ 全天候自动处理在线订单
✓ 博学专业的产品和技术支持
✓ 快速可靠的(再)订购
特点
- 在血清存在的情况下进行高效转染
- 只需使用少量DNA 即可达到高效转染
- 高转染效率而且较低的DNA使用量
- 适合高通量筛选
产品详情
绩效
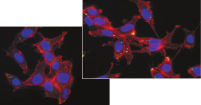
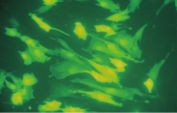
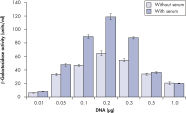
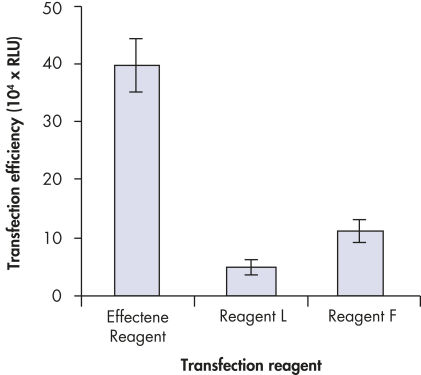
Effectene Transfection Reagent 的操作流程简单, 转染质粒DNA比用其他的试剂获得的转染效率高(参见" High transfection efficiencies using Effectene Reagent")。Effectene Transfection Reagent适用于带有寡核苷酸的敏感细胞系的转染(参见" Transfection of oligonucleotides using Effectene Reagent")。特别适用于原代细胞(参见" 40% transfection efficiency in primary cells")。 使用Effectene Transfection Reagent已成功转染了许多细胞系和原代细胞。有针对不同细胞类型的转染方案。细胞毒性低,因为可在血清存在的情况下使用Effectene Transfection Reagent进行转染且DNA用量低(参见" Serum and DNA quantity vs. transfection efficiency")。
DNA重组技术应用于药物研发领域,导致对高通量转染需求增加。使用Effectene Transfection Reagent进行转染DNA用量低,手动操作少。此外,无需移除转染复合物,使该试剂高度适用于高通量筛选。Effectene Transfection Reagent适用于批量转染。
查看图表
原理
Effectene Transfection Reagent是一种全新的非脂质体的脂类转染剂,将DNA 与浓缩增强子和优化的缓冲液混合使用以达到高效转染。浓缩增强子先聚合DNA分子,Effectene Transfection Reagent随后用阳离子脂质包围聚合的DNA分子。采用一种特殊有效的方法将DNA转入真核细胞。这种特性保证了复杂复合物转染的可重现性。
程序
Effectene流程分为两步。将DNA与浓缩增强子混合后,加入Effectene Transfection Reagent形成复合物,这一步只需2–5分钟。然后加入Effectene Transfection Reagent,与混合物一起反应5–10分钟以形成Effectene–DNA复合物。复合物与培养基混合(可以含有血清和抗体),直接加入到细胞中。培养细胞至成熟,分析基因表达(参见" Effectene transfection procedure")。
查看图表
应用
Effectene Transfection Reagent适用于多种类型细胞的瞬时和稳定转染。
辅助数据和图表
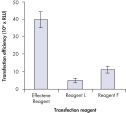
High transfection efficiencies using Effectene Reagent.
Specifications
| Features | Specifications |
|---|---|
| Applications | RNAi studies, gene expression studies, high-throughput transfections |
| Technology | Non-liposomal lipid formulation in conjonction with a DNA-condensing enhancer |
| Number of possible transfections | 160 transfections in 12-well plates / 1 ml reagent |
| Cell type | Eukaryotic cells (primary cells and sensitive cells) |
| Features | Non-liposomal lipid formulation, minimal cytotoxicity |
| Transfection type | Transient and stable transfection |
| Controls | Not included |
| Nucleic acid | DNA |