RNeasy Lipid Tissue Mini Kit
从富含脂肪的组织和其他类型组织中纯化至多100 µg的总RNA
从富含脂肪的组织和其他类型组织中纯化至多100 µg的总RNA
✓ 全天候自动处理在线订单
✓ 博学专业的产品和技术支持
✓ 快速可靠的(再)订购
Cat. No. / ID: 74804
✓ 全天候自动处理在线订单
✓ 博学专业的产品和技术支持
✓ 快速可靠的(再)订购
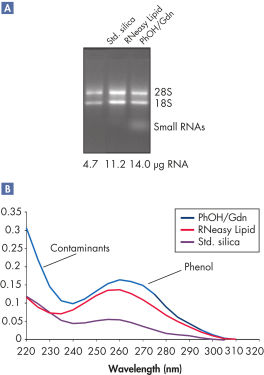
RNeasy Lipid Tissue Kits适用于富含脂肪的组织,如脑和脂肪组织。方便RNeasy Lipid Tissue实验方案结合了优化的苯酚/胍的裂解方法以及经验证的RNeasy硅胶膜纯化方法,能纯化得到高品质的总RNA。QIAzol Lysis Reagent结合了有机提取与离液破碎的方法,具有很高的裂解效率。RNeasy技术适用于大于200 nt RNA,因此RNA产物不包括5S rRNA、tRNA或其他小分子量RNAs,产物占细胞内总RNA的15–20%。
RNeasy Lipid Tissue Kits可简单有效的纯化高品质RNA,得到的RNA适用于多种下游应用,包括芯片分析和real-time RT-PCR。
| Features | Specifications |
|---|---|
| Applications | PCR, real-time PCR, microarray |
| Sample amount | 10–100 mg |
| Yield | 2.4–10 mg |
| Technology | Silica technology |
| Processing | Manual |
| Purification of total RNA, miRNA, poly A+ mRNA, DNA or protein | RNA |
| Elution volume | 30–100 µl |
| Main sample type | Fatty tissue samples |
| Time per run or per prep | 45 minutes |
| Format | Spin column |