RT2 Profiler PCR Arrays
用于可靠、灵敏的基因表达谱分析
用于可靠、灵敏的基因表达谱分析
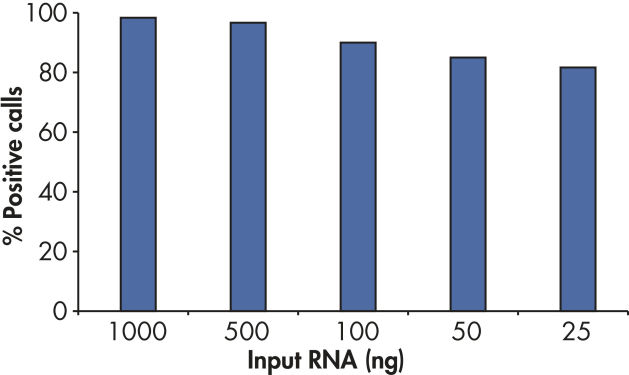
RT2 First Strand Kit灵敏度高,每个芯片使用至少1 ng或至多5 µg总RNA可获得高于80%的阳性信号率(参见" Positive results with as little as 25 ng RNA")。
整个PCR芯片体系与技术上的重复性、批次和仪器有很高的相关性,平均相关系数>0.99,确保对生物样本间的表达差异进行可靠的检测(参见" High reproducibility among different users")。
PCR芯片体系使用高品质RNA,可在预期位置形成单条带,无引物二聚体或其他二级产物,由此可获得高度准确的real-time PCR结果(参见" A single gene-specific product in every reaction")。
对多种样本的多个基因进行准确的基因表达比较,需要PCR芯片技术具有统一的PCR扩增效率。将专有的引物设计法则与严格的引物分析检测相结合,确保PCR芯片的每次引物分析都具有高性能(参见" PCR arrays yield highly accurate results")。
RT2 Profiler PCR Arrays是分析一组特定基因表达的可靠工具。每个96孔板、384孔板或100孔盘PCR芯片都包含SYBR® Green优化的引物分析,可对相关的通路或疾病相关的基因进行细致的研究。RT2 Profiler PCR Arrays也可针对您特定的研究兴趣对一些基因作定制化分析。高品质引物设计和RT2 SYBR® Green qPCR Mastermix规格使PCR芯片可在统一的循环条件下同时扩增96或384个不同的基因特异性产物。
这种整合使RT2 Profiler PCR Array具有高特异性和扩增效率,可获得准确的SYBR® Green real-time PCR结果。PCR芯片在任何研究实验室都易于使用。
RT2 Profiler PCR Arrays具有足够高的敏感度,可使用从常规样本(0.1–5 µg RNA)、FFPE样本和小样本(1–100 ng RNA)制备的RNA。
只需将cDNA模板与合适的即用型PCR预混液混合,等体积加入到同一个孔板的每个孔中,然后即可运行real-time PCR循环程序(参见" Simple procedure")。RT2 Profiler PCR Arrays可与所有QIAGEN、ABI、Bio-Rad、Eppendorf、Roche和Stratagene的仪器兼容。
RT2 Profiler PCR Arrays有96孔板、384孔板和100孔盘等规格,可检测84或370个与疾病或通路相关的基因,外加5个管家基因。每个RT2 Profiler PCR Array还包括以下对照因素:
可使用易用的基于Excel的数据分析模板或基于网络的软件进行数据分析。数据分析基于ΔΔCT法,原始数据可对每个管家基因标准化。
RT2 PCR Profiler Arrays可用于生物学和医学研究的各个领域,包括: