QIAGEN Large-Construct Kit
纯化至多50 μg BAC、PAC和P1 DNA或至多200 μg的柯斯质粒DNA和非基因组DNA
纯化至多50 μg BAC、PAC和P1 DNA或至多200 μg的柯斯质粒DNA和非基因组DNA
✓ 全天候自动处理在线订单
✓ 博学专业的产品和技术支持
✓ 快速可靠的(再)订购
QIAGEN Large-Construct Kit提供重力流和阴离子交换柱,用于纯化大分子量的DNA。独特的ATP-dependent外切酶消化步骤,确保特异性去除基因组DNA。产物DNA的纯度相当于经过两次CsCl密度梯度离心得到的纯度,适用于转染级别的应用。
QIAGEN Large-Construct Kit使用经优化的重力流操作,获得的DNA的纯度显著高于其他常规方法获得的DNA的纯度。独特的ATP-dependent外切酶消化步骤,确保特异性去除基因组DNA。
QIAGEN Large-Construct Kit含有的QIAGEN-tips中独特的阴离子交换树脂专为核酸纯化设计。其出色的核酸分离特性可纯化得到高纯度DNA,相当于经过两次氯化铯梯度离心得到的纯度。预装的QIAGEN-tips(参见" Anion-exchange tips")运用重力沉降原理,减少了质粒制备过程所需的手工操作时间。整个QIAGEN质粒纯化系统不使用苯酚、氯仿、溴乙锭和氯化铯等有毒物质,最大程度减小了对使用者及环境的危害。
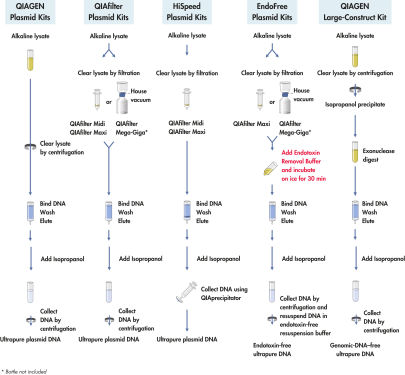
使用碱裂解法裂解多达500 ml的培养液后(参见" QIAGEN Plasmid Kit procedures"),使用试剂盒中的ATP-dependent外切酶进行消化,确保特异性去除基因组DNA及缺损的高分子量DNA。之后将样本上样至阴离子交换柱,在适当的低盐浓度和pH值条件下,质粒DNA与树脂特异性结合。用中等盐浓度的缓冲液洗涤,去除RNA、蛋白质、代谢物和其他小分子的杂质。用高盐缓冲液洗脱已去除基因组DNA的纯化质粒DNA。加入异丙醇使DNA浓缩和脱盐,之后离心收集。
QIAGEN Large-Construct Kit纯化获得的DNA适用于各种下游应用,包括:
| Features | Specifications |
|---|---|
| Plasmid type | BAC, PAC, P1, cosmid DNA |
| Applications | Subcloning, transfection, sequencing etc. |
| Processing | Manual (centrifugation) |
| Culture volume/starting material | <500 ml culture volume |
| Samples per run (throughput) | 1 sample per run |
| Technology | Anion-exchange technology |
| Time per run or prep per run | 280 min |
| Yield | <150 ug |