QIAamp DSP Virus Kit
从人类血清或血浆中纯化病毒核酸
从人类血清或血浆中纯化病毒核酸
✓ 全天候自动处理在线订单
✓ 博学专业的产品和技术支持
✓ 快速可靠的(再)订购
Cat. No. / ID: 60704
✓ 全天候自动处理在线订单
✓ 博学专业的产品和技术支持
✓ 快速可靠的(再)订购
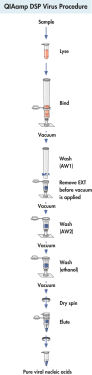
QIAamp DSP Virus Kit采用成熟和方便的QIAamp技术同时纯化病毒DNA和RNA。
使用QIAamp DSP Virus Kit纯化的病毒核酸可即用于各种敏感的下游应用,如基于酶的扩增或其他修饰,包括:PCR和RT-PCR。
QIAamp DSP Virus Kit采用成熟和方便的QIAamp技术同时纯化病毒DNA和RNA。QIAamp硅胶膜特异性结合裂解样品中的核酸,其他裂解物通过真空操作去除。经过有效地洗涤去除污染物,然后洗脱DNA,洗脱体积为20 µl或60 µl。
QIAamp DSP Virus Kit可纯化各种病毒核酸,与各种上游样品收集系统和下游应用兼容。
| Features | Specifications |
|---|---|
| Applications | PCR, RT-PCR, LCR |
| Elution volume | 20 µl, 60 µl |
| Main sample type | Serum, plasma |
| CE/FDA/IVD compatible | CE/IVD |
| Format | Sample tubes |
| Processing | Manual (vacuum) |
| Purification of total RNA, miRNA, poly A+ mRNA, DNA or protein | Viral DNA, viral RNA |
| Sample amount | 500 µl |
| Technology | Silica technology |
| Yield | Varies |