QIAsymphony Virus/Bacteria Kits
从1–96个样本中自动纯化病毒核酸或细菌DNA
从1–96个样本中自动纯化病毒核酸或细菌DNA
QIAsymphony Virus/Bacteria Kit可在QIAsymphony SP核酸提取纯化分析仪上,从多种类型样本中自动纯化病毒核酸和细菌DNA。例如,可以从血浆、血清、脑脊液、呼吸道样本、尿常规样本、泌尿生殖道拭子和运输介质中纯化核酸。试剂盒有小型和中型规格,对应样本体积200 μl和至多1000 μl。经优化的实验方案提供高产量高纯度的核酸,和灵活的洗脱体积,因而可以根据每个下游应用,优化调整核酸浓度。新型的预装试剂条减少了工作站设置时间,并减少了手动操作。条形码阅读器进行试剂全程跟踪。
纯化操作流程的目的在于确保安全、可重复的处理传染性样本,包括4个步骤:裂解、结合、洗涤和洗脱。用户可以选择不同的洗脱体积,取决于实验方案。在QIAsymphony SP核酸提取纯化分析仪上可完成所有纯化流程的步骤。每次运行至多可以处理96个样本。
| 实验方案 | 试剂盒 | 样本材料 | 处理体积 |
|---|---|---|---|
| Cellfree 200 | QIAsymphony Virus/Bacteria Mini Kit (192) | 血浆、血清和脑脊液 | 200 μl |
| Cellfree 500 | QIAsymphony Virus/Bacteria Midi Kit (96) | 血浆、血清和脑脊液 | 500 μl |
| Cellfree 1000 | QIAsymphony Virus/Bacteria Midi Kit (96) | 血浆、血清和脑脊液 | 1000 μl |
| Complex 200 | QIAsymphony Virus/Bacteria Mini Kit (192) | 呼吸道样本(BAL、干拭子、传输介质、呼出物、痰)和泌尿生殖系统的样品(尿液、运输培养基) | 200 μl |
| Complex 400 | QIAsymphony Virus/Bacteria Midi Kit (96) | 呼吸道样本(BAL、干拭子、传输介质、呼出物、痰)和泌尿生殖系统的样品(尿液、运输培养基) | 400 μl |
| Complex 800 | QIAsymphony Virus/Bacteria Midi Kit (96) | 呼吸道样本(BAL、干拭子、传输介质、呼出物、痰)和泌尿生殖系统的样品(尿液、运输培养基) | 800 μl |
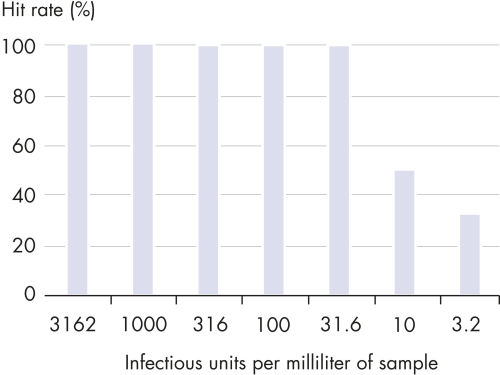
QIAsymphony Virus/Bacteria Kit能够灵敏的检测多种DNA和RNA病毒以及细菌病原体。产量呈线性,准确的定量分析高和低的病毒或者细菌滴度。高品质的核酸可即用于各种下游应用,包括定量real-time PCR或者RT-PCR等敏感的应用。
| Features | Specifications |
|---|---|
| Applications | PCR, real-time PCR |
| Elution volume | 60-110 µl |
| Purification of total RNA, miRNA, poly A+ mRNA, DNA or protein | Purification of viral RNA and DNA and bacterial DNA |
| Input volume | 800 µl (Pathogen Complex Protocol); 1000 µl (Virus Cellfree Protocol) |
| Technology | Magnetic particles |
| Sample types | Serum, plasma, CSF, cell-free body fluids, fresh or frozen material (body fluids, swabs, secretions, tissues, stool specimens) |