QIAsymphony DSP DNA Kits
适用于1–96个样本DNA的自动化纯化
QIAsymphony DSP DNA Kits
适用于1–96个样本DNA的自动化纯化

QIAsymphony DSP DNA Kits系列试剂盒可配套QIAsymphony SP仪器用于从人类全血、白膜层、组织、FFPE组织、培养的细胞及细菌等样本中进行DNA的自动化提取,也可用于体外诊断实验所需的人类全血中病毒DNA的自动化提取。该类试剂盒可分为小量和中量提取两种规格,适用于体积从200 µl到1000 µl的起始样本。对于组织、FFPE组织、培养的细胞及细菌样本,手工操作的样本预处理过程是必要的。经优化的一系列操作步骤可帮助从样本中获得较高产量的纯化DNA,灵活的洗脱体积为下游多种应用所需的不同DNA浓度优化提供了可能。创新的预装试剂条则能够最大程度的减少了实验设置和手工操作的时间。附带的条形码扫描功能可用于相关试剂的全程追踪。
QIAsymphony DSP DNA Kits can be used to purify total DNA from human whole blood, buffy coat, tissues and FFPE tissues, as well as viral DNA from human whole blood.
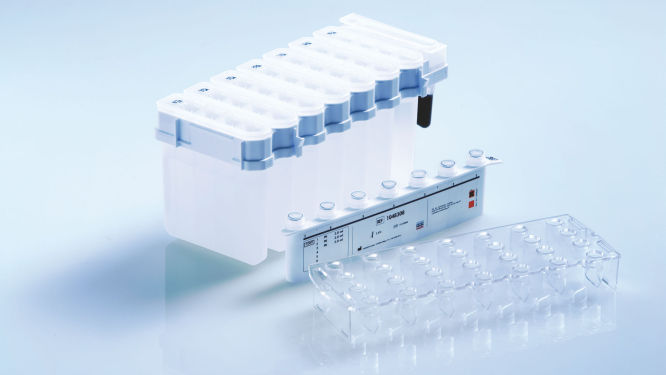
QIAsymphony DSP DNA Kits combine the speed and efficiency of silica-based purification of DNA with the convenient handling of magnetic particles (see figure " Schematic of the QIAsymphony SP principle"). Innovative, ready-to-run reagent cartridges are prefilled with all reagents required for the purification procedure and are automatically opened by the QIAsymphony SP, which helps to minimize the risk of environmental contamination (see figure " Prefilled, sealed reagent cartridges"). Worktable setup is rapid, which saves you valuable time. Simply place up to 2 reagent cartridges in the consumables drawer. The reagent cartridges can be either from the same kit or from different kits, enabling different purification procedures to be performed within the same run of 96 samples. Reagent volumes only for the selected number of samples are used, giving you complete cost control.
The purification procedure is designed to ensure safe and reproducible handling of potentially infectious samples, and comprises 4 steps: lyse, bind, wash, and elute (see figure "QIAsymphony DSP DNA procedure"). Optimized protocols provide high quality nucleic acids with efficient removal of proteins, nucleases, and other impurities. Nucleic acids purified with QIAsymphony DSP DNA Kits are ready for direct use in downstream applications such as amplification. The user can choose between different elution volumes.
QIAsymphony DSP DNA Kits系列试剂盒纯化得到的高品质核酸可直接适用于下游的多种应用,如测序或是诸如实时定量PCR、RT-PCR等较为灵敏的检测实验。