Features
- Prefilled reagent cartridges, sufficient for 2 x 352 sample preparations
- High reproducibility through standardized processing
- Processes up to 88 samples, in 24 batches per run, in less than 2.5 hours
- Bar codes for complete tracking of samples and reagents
- For in vitro diagnostic use
Product Details
The QIAsymphony DSP HPV Media Kit, in combination with the QIAsymphony SP, provides sample extracts that are ready for automated testing using the digene HC2 High-Risk HPV DNA Test with the Rapid Capture System. This kit uses QIAsymphony magnetic particle technology for automated isolation of human cervical cells stored in liquid-based cytology (LBC) media.
Principle
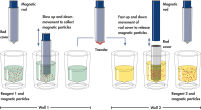
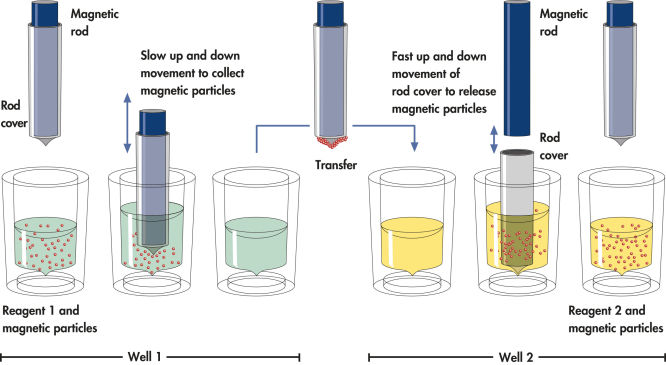
QIAsymphony DSP HPV Media technology combines the speed and efficiency of pH-driven anion exchange chromatography with the convenient handling of magnetic particles. Ready-to-run reagent cartridges are prefilled with all the reagents required for the sample preparation procedure and are automatically opened by the QIAsymphony SP ( Schematic of QIAsymphony SP principle), which helps to minimize the risk of environmental contamination.
See figures
Procedure
The sample preparation procedure is designed to ensure safe and reproducible handling of potentially infectious samples, and comprises binding and recovery of sample extracts. Worktable setup is rapid, which saves valuable time. Simply check the waste drawer, load consumables, and load 2 reagent cartridges. Reagent volumes are used only for the selected number of samples, allowing complete cost control.
| Protocol | Sample type | Volume processed |
|---|---|---|
| PC2500_HC2_V1_DSP | PreservCyt samples | 2.5 mL |
| SP800_HC2_V1_DSP | SurePath specimens/post-gradient cell pellet samples | 800 µL |
Applications
The QIAsymphony DSP HPV Media Kit, when automated on the QIAsymphony SP, enables the sample preparation of specimens that have been collected in liquid-based cytology media, such as PreservCyt Solution (Hologic) or SurePath Preservative Fluid.
Supporting data and figures
Schematic of the QIAsymphony SP principle.
Schematic of the QIAsymphony SP principle.
Resources
Safety Data Sheets (1)
Certificates of Analysis (1)