Transfection grade의 plasmid 혹은 cosmid DNA을 10 mg까지 분리 정제할 수 있습니다
✓ 연중무휴 하루 24시간 자동 온라인 주문 처리
✓ 풍부한 지식과 전문성을 갖춘 제품 및 기술 지원
✓ 신속하고 안정적인 (재)주문
QIAGEN Plasmid Mega Kit (5)
Cat. No. / ID: 12181
✓ 연중무휴 하루 24시간 자동 온라인 주문 처리
✓ 풍부한 지식과 전문성을 갖춘 제품 및 기술 지원
✓ 신속하고 안정적인 (재)주문
특징
- CsCl 농도구배 원심분리 두 번 한 것과 같은 동일한 순도
- Transfection grade plasmid DNA 의 재현성 있는 yield.
- Ethidium bromide, phenol, chloroform, 또는 CsCl 불필요
- 경제적인 preparation이 가능함
제품 세부 정보
QIAGEN Plasmid Kits provide gravity-flow, anion-exchange tips for purification of transfection-grade plasmid DNA. The QIAGEN Plasmid Giga Kit delivers DNA yields of up to 10 mg. Lysate clearing and isopropanol precipitation are achieved by centrifugation.
성능
원리
The unique anion-exchange resin in QIAGEN-tips is developed exclusively for the purification of nucleic acids. Its exceptional separation properties result in DNA purity equivalent or superior to that obtained by two successive rounds of CsCl gradient centrifugation. Prepacked QIAGEN-tips operate by gravity flow and never run dry, minimizing the hands-on time required for plasmid preparation. The entire QIAGEN plasmid purification system avoids the use of toxic substances such as phenol, chloroform, ethidium bromide, and CsCl, minimizing hazard both to the user and the environment.
절차
With QIAGEN Plasmid Kits, bacterial lysates are cleared by centrifugation. The cleared lysate is then loaded onto the anion-exchange tip where plasmid DNA selectively binds under appropriate low-salt and pH conditions. RNA, proteins, metabolites, and other low-molecular-weight impurities are removed by a medium-salt wash, and pure plasmid DNA is eluted in high-salt buffer (see flowchart "QIAGEN Plasmid Kit procedures"). The DNA is concentrated and desalted by isopropanol precipitation and collected by centrifugation.
응용 분야
Plasmid DNA purified with QIAGEN Plasmid Kits is highly suitable for use in applications, such as:
- Transfection
- Cloning
- PCR
- In vitro transcription
지원되는 데이터 및 수치
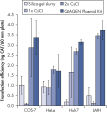
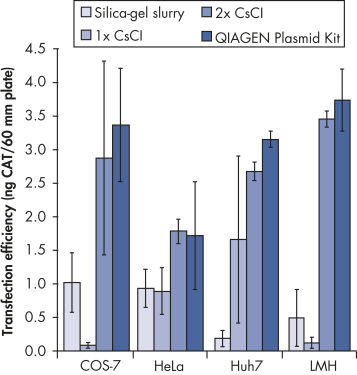
Transfection efficiency versus plasmid purification method.
Specifications
| Features | Specifications |
|---|---|
| Technology | Anion-exchange technology |
| Culture volume/starting material | 3 ml–5 liters culture volume |
| Yield | <20 µg to <10 mg |
| Processing | Manual (gravity flow) |
| Samples per run (throughput) | 1 sample per run |
| Time per run or prep per run | 80–320 min |
| Applications | Transfection, cloning, sequencing, capillary sequencing etc. |
| Plasmid type | High-copy, low-copy, cosmid DNA |