BioRobot 9604 workstation을 이용하여 cell-free body fluids에서 viral nucleic acids를 자동으로 분리 정제할 수 있습니다
Products

QIAamp Virus BioRobot 9604 Kit (12)
Cat. No. / ID: 965662
특징
- High-quality의 신속한 분리 정제, ready-to use viral DNA and RNA
- Organic extraction 또는 alcohol precipitation 불필요
- 효율이 높고 결과가 일정함
- 정확한 downstream applications을 위해 inhibitor와 contaminant를 완벽히 제거함
제품 세부 정보
The QIAamp Virus BioRobot 9604 Kit provides automated viral nucleic acid purification on the BioRobot 9604 using proven QIAamp technology. The fully automated procedure requires less than 2.5 hours for up to 96 samples, including bar code reading and complete process documentation, with no hands-on time during the run.
성능
QIAamp 96 plates provide well-to-well uniformity in RNA and DNA recovery for reliable results in downstream applications. QIAamp technology yields viral RNA and DNA from cell-free body fluids ready to use in PCR and blotting procedures.
원리
No phenol–chloroform extraction is required. Nucleic acids bind specifically to the QIAamp silica-gel membrane while contaminants pass through. PCR inhibitors, such as divalent cations and proteins, are completely removed in efficient wash steps, leaving pure nucleic acids to be eluted in a buffer provided with the kit.
절차
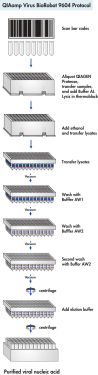
Optimized buffers lyse samples, stabilize nucleic acids, and enhance selective nucleic acid adsorption to the QIAamp membrane. Alcohol is added and lysates loaded onto the QIAamp 96-well plate. Wash buffers are used to remove impurities and pure, ready-to-use nucleic acid is then eluted in a low-salt buffer using the BioRobot 9604 (see flowchart " Protocol").
Highly pure, viral RNA and DNA can be isolated from 96 cell-free fluid samples in less than 2.5 hours. This kit can be used to isolate nucleic acids from human and animal viruses. QIAamp sample preparation technology is fully licensed.
그림 참조
응용 분야
The QIAamp Virus BioRobot 9604 Kit provides proven QIAamp technology in a convenient 96-well format for automated high-throughput purification of viral nucleic acids on the BioRobot 9604. The kit provides purification of nucleic acids from 200 µl or less of samples, including:
- Plasma
- Serum
- CSF
- Other cell-free body fluids
- Cell-culture supernatants
지원되는 데이터 및 수치
QIAamp Virus BioRobot 9604 protocol.
Specifications
| Features | Specifications |
|---|---|
| Applications | PCR, blotting |
| For automated processing | BioRobot 9604 Workstation |
| Main sample type | Serum, plasma |
| Elution volume | 50 µl |
| Format | 96-well plates |
| Processing | Automated |
| Purification of total RNA, miRNA, poly A+ mRNA, DNA or protein | Viral DNA, viral RNA |
| Technology | Silica technology |
| Sample amount | <200 µl |
| Time per run or per prep | <2.5 hours |
| Yield | >90% recovery |

