모든 applications에 최적의 amplication이 가능합니다
HotStarTaq DNA Polymerase (250 U)
Cat. No. / ID: 203203
특징
- Hot start tag 사용으로 non-specific amplification을 줄임
- Optimization 최소화 — 최소의 시간과 비용
- 쉬운 사용방법 — 상온에서 간단하게 reaction setup이 가능함
제품 세부 정보
HotStarTaq DNA Polymerase uses a chemically mediated hot start that, unlike, antibody-mediated systems, leads to complete inactivation of the polymerase until the initial heat activation step at the start of PCR. HotStarTaq DNA Polymerase is supplied with the unique QIAGEN PCR Buffer, which minimizes nonspecific amplification products, primer dimers, and background. Q-Solution, a novel additive that enables efficient amplification of "difficult" (e.g., GC rich) templates, is also provided.
성능
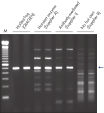
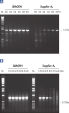
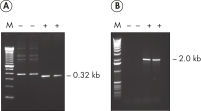
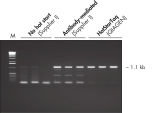
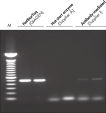
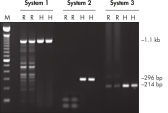
Each lot of HotStarTaq DNA Polymerase is subjected to a comprehensive range of quality control tests, including a stringent PCR specificity and reproducibility assay in which low-copy targets are amplified. HotStarTaq DNA Polymerase outperformed kits tested from other suppliers and ensures high specificity and superior performance in hot-start PCR (see figures "Higher specificity with different primer–template systems" and " Superior performance" and table). The innovative PCR buffer provided with the kit ensures specificity over a wide range of PCR conditions, minimizing the need for optimization (see figures " Wide annealing temperature window" and " Tolerance to variable magnesium concentration"). Suboptimal PCR can be improved with Q-Solution, also provided with the kit (see figure " Amplification of difficult templates"). Together, these components ensure specific amplification in a range of applications (see figure " Effect of hot start on RT-PCR performance" and " Highly sensitive single-cell PCR").
| HotStarTaq DNA Polymerase | Hot-start enzyme from Supplier AII | Antibody-mediated | Manual | Wax barrier | |
|---|---|---|---|---|---|
| Specific amplification | ++ | + | + | +/– | +/– |
| Minimal PCR optimization | ++ | +/– | +/– | – | – |
| Easy to use | ++ | ++ | + | – | – |
HotStarTaq DNA Polymerase specifications
Concentration: 5 units/µl
Recombinant enzyme: Yes
Substrate analogs: dNTP, ddNTP, dUTP, biotin-11-dUTP, DIG-11-dUTP, fluorescent-dNTP/ddNTP
Extension rate: 2–4 kb/min at 72°C
Half-life: 10 min at 97°C ; 60 min at 94°C
Amplification efficiency: ≥105 fold
5'–>3' exonuclease activity: Yes
Extra A addition: Yes
3'–>5' exonuclease activity: No
Contaminating nucleases: No
Contaminating RNases: No
Contaminating proteases: No
Self-priming activity: No
그림 참조
원리
HotStarTaq DNA Polymerase, a modified form of Taq DNA Polymerase, provides high specificity in hot-start PCR. The kit includes an innovative dual-cation PCR buffer, Q-Solution, and MgCl2.
HotStarTaq DNA Polymerase
HotStarTaq DNA Polymerase is supplied in an inactive state and has no polymerase activity at ambient temperatures. This prevents extension of nonspecifically annealed primers and primer dimers formed at low temperatures during PCR setup and the initial PCR cycle (see figures " Superior performance in hot-start PCR" and "Higher specificity with different primer–template systems"). HotStarTaq DNA Polymerase is activated by a 15-minute incubation at 95°C, which can be incorporated into any existing thermal-cycler program.
QIAGEN PCR Buffer
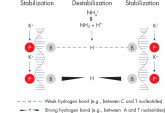
QIAGEN PCR Buffer maintains specific amplification in every cycle of PCR by promoting a high ratio of specific-to-nonspecific primer binding during the annealing step in each PCR cycle (see figure " Increased specificity of primer annealing"). Owing to a uniquely balanced combination of KCl and (NH4)2SO4, the buffer provides stringent primer-annealing conditions over a wider range of annealing temperatures and Mg2+ concentrations than conventional PCR buffers. Optimization of PCR by varying the annealing temperature or the Mg2+ concentration is therefore often minimal or not required (see figures " Wide annealing temperature window" and " Tolerance to variable magnesium concentration").
Q-Solution
Q-Solution, an innovative PCR additive that facilitates amplification of difficult templates by modifying the melting behavior of DNA, is also provided with HotStarTaq DNA Polymerase. This unique reagent improves suboptimal PCR caused by templates that have a high degree of secondary structure or with GC-rich templates (see figure " Amplification of difficult templates"). Unlike other commonly used PCR additives such as DMSO, Q-Solution is used at just one working concentration, is nontoxic, and PCR purity is guaranteed. Adding Q-Solution to the PCR does not compromise PCR fidelity.
그림 참조
절차
응용 분야
HotStarTaq DNA Polymerase is suitable for a wide variety of applications, including challenging applications, such as amplification of:
- Complex genomic templates
- Complex cDNA templates (e.g., RT-PCR)
- Very low-copy targets (e.g., single-cell PCR)
- Reactions with multiple primer pairs
지원되는 데이터 및 수치
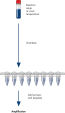
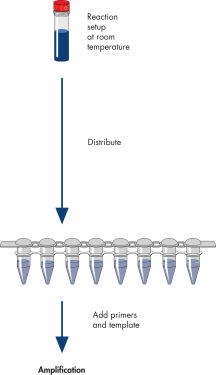
HotStarTaq procedure.
Specifications
| Features | Specifications |
|---|---|
| Applications | PCR, RT-PCR, Complex genomic templates, very low-copy targets |
| With/without hotstart | With hotstart |
| Reaction type | PCR amplification |
| Sample/target type | Genomic DNA and cDNA |
| Real-time or endpoint | Endpoint |
| Enzyme activity | 5' -> 3' exonuclease activity |
| Mastermix | No |
| Single or multiplex | Single |