HiSpeed Plasmid Kits
750 µgまでのトランスフェクショングレードのプラスミドあるいはコスミドDNAの超高速精製
750 µgまでのトランスフェクショングレードのプラスミドあるいはコスミドDNAの超高速精製
✓ オンライン注文による24時間年中無休の自動処理システム
✓ 知識豊富で専門的な製品&テクニカルサポート
✓ 迅速で信頼性の高い(再)注文
Cat. No. / ID: 12643
✓ オンライン注文による24時間年中無休の自動処理システム
✓ 知識豊富で専門的な製品&テクニカルサポート
✓ 迅速で信頼性の高い(再)注文
HiSpeed Plasmid Kits は超高速、ラージスケールでの陰イオン交換ベースのプラスミド調製を行ない、遠心操作は必要ありません。精製したDNAは、CsCl 密度勾配遠心法を2 回行なって得られる精製グレードに匹敵し、トランスフェクション等のアプリケーションに最適です。
HiSpeed Plasmid Kits は、真空マニホールドなしでの高速の大量プラスミド調製用にQIAfilter Cartridge、HiSpeed TipおよびQIAprecipitatorモジュールを備えています。シリンジフォーマットのQIAfilterとQIAprecipitatorモジュールにより従来の陰イオン交換調製法における遠心操作の必要がなくなり、プラスミド精製がより迅速で簡単になりました。150~250 ml の培養液から最高750 µg(Maxi)、または50~150 ml の培養液から最高200 µg(Midi)の高コピープラスミドDNAを精製することができます(培養液の容量はプラスミドコピー数、挿入DNAのサイズ、宿主株、培養液に依存)。HiSpeed Tip は、流速が早くなり、プラスミド精製のためのDNA結合、洗浄および溶出ステップがより迅速に行なえます。
HiSpeed Tipsの画期的な陰イオン交換樹脂は核酸精製用に特化して開発されています。本製品の優れた核酸分離能力により、CsCl 密度勾配遠心分離法を2回行なって得たDNAの純度に匹敵、あるいはそれ以上の純度のDNAが調製されます。
QIAfilter Cartridges(図" QIAfilter Cartridge") は、バクテリア細胞のアルカリ溶解後に行なう遠心操作による清澄化に代わる方法として開発された特別なフィルターです。QIAfilter Cartridges はSDS沈殿物を完全に取り除くばかりではなく、遠心操作に必要な時間よりずっと早くバクテリアライセートを清澄化します。充填済みHiSpeed Tipsはオープンカラムで操作し、乾燥することはなく、プラスミド調製に必要なマニュアルでの作業時間を短縮できます。
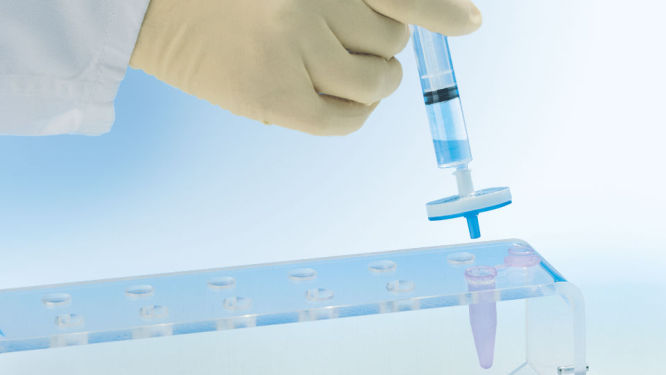
ユニークな QIAprecipitatorモジュール (図" QIAprecipitatorモジュール")により、イソプロパノールで沈殿させたDNAを収集するために従来の遠心操作の必要はなくなりました。沈殿したDNA は、QIAprecipitator モジュールで捕獲され、イソプロパノールやバッファーは流出します。このDNAはTEバッファーあるいは水によりQIAprecipitator からマイクロ遠心チューブに簡単に溶出されます。このモジュールにより、遠心操作後に上清を除去する際に起こり得るペレットを失うリスクもなくなりました。
| 特徴 | HiSpeed Plasmid Maxi Kit | HiSpeed Plasmid Midi Kit | |
| Applications | Transfection, cloning sequencing, gene silencing | Transfection, cloning sequencing, gene silencing | |
| Culture volume/starting material | 150 µl – 250 ml culture volume | 50 µl – 150 ml culture volume | |
| Plasmid type | High-copy, cosmid DNA | High-copy, cosmid DNA | |
| Processing | Manual (filtration) | Manual (filtration) | |
| Sample per run | 1 sample per run | 1 sample per run | |
| Technology | Anion-exchange technology | Anion-exchange technology | |
| Time per run | 60 min | 45 min | |
| Yield | <750 µg | <200 µg | |
中和したバクテリアライセートをQIAfilter Cartridge 中でインキュベートし、ろ過によりすばやく清澄化し、プラスミドDNA精製のためにろ液をHiSpeed Tip にアプライします(図"QIAGEN Plasmid Kit 操作の比較")。溶出したDNAをイソプロパノールと混合し、添付のシリンジを利用してQIAprecipitator モジュールにアプライします。濃縮そして脱塩されたDNA は、TEバッファーあるいは水でQIAprecipitator から直接マイクロ遠心チューブに溶出されます。
HiSpeed Plasmid Kits で精製したDNA は、クローニング、シークエンシング、トランスフェクション、遺伝子サイレンシングを含む幅広いアプリケーションに最適です