✓ オンライン注文による24時間年中無休の自動処理システム
✓ 知識豊富で専門的な製品&テクニカルサポート
✓ 迅速で信頼性の高い(再)注文
RNeasy Plant Mini Kit (50)
Cat. No. / ID: 74904
✓ オンライン注文による24時間年中無休の自動処理システム
✓ 知識豊富で専門的な製品&テクニカルサポート
✓ 迅速で信頼性の高い(再)注文
特徴
- 30分で高品質のトータルRNA
- フェノール/クロロホルム抽出不要
- CsCl 勾配遠心分離、LiCl やエタノール沈殿は不要
- 非常に高いRNA回収率
- あらゆるダウンストリームアプリケーションに即使用可能なRNA
製品詳細
RNeasy Plant Mini Kitには、粘性の高い植物や菌類ライセートのホモジナイズとろ過用のQIAshredder Spin Column、シリカゲルメンブレンテクノロジーを用いた高品質RNA精製用(100 µgまで)のRNeasy Spin Columnが含まれています。精製はQIAcube上で自動化可能です。また、本キットは植物サンプルの効率的な破砕とホモジナイゼーションを実現するTissueRuptorあるいはTissueLyserシステムと組み合わせて使用することも可能です。
パフォーマンス
RNeasy Plant Mini Kitは、様々な植物や菌類サンプルからのトータルRNAの精製に最適です。サンプルサイズは、10~100 mgの組織、または100~1 x 107個の細胞に対応しています(表"RNeasy Plant Mini Kitで調製したサンプルの例")。スピンカラムは最大100 µgのRNAを結合できます。100 mgの植物組織から得られる一般的なRNA収量は25~65 µgですが、RNA量はサンプルの発達段階および増殖条件により異なる可能性があります(表"100 mgの組織から得られる収量")。
| 植物 | 糸状菌類 |
|---|---|
| Anemone sp. Arabidopsis thaliana1 Begonia sp. Beta vulgaris (サトウダイコン)2 Chlamydomonas reinhardii(単細胞藻) Chrysanthemum Clarkia spp.3 Daucus carota (ニンジン)4 Diascia sp. Dendranthema sp. Euglena gracilis(単細胞藻) Funaria hygrometrica(コケ) Hordeum vulgare(オオムギ) Lycopersicon esculentum(トマト)5 Malus sp. (リンゴ)6 Nicotiana tabacum(タバコ) Oryza sativa(イネ) Pelargonium sp.(ゼラニウム) Petroselinum crispum(パセリ)7 Pisum sativum(エンドウ) Prunus sp.(サクラ)6 Ranunculus sp. Ribes nigrum(クロフサスグリ) Riccia fluitans(ゼニゴケ) Sinapis arvensis (カラシ) Solanum tuberosum(ジャガイモ)8 Spinacia oleracea(ホウレンソウ) Surfinia sp. Triticum aestivum(小麦) Vitis sp.(ブドウ)6 Zea mays(トウモロコシ) | Acremonium crysogenum9 Fusarium avenaceum9 |
| サンプルタイプ | 収量 |
|---|---|
| アラビドプシスの葉 | 35 µg |
| トウモロコシの葉 | 25 µg |
| トマトの葉 | 65 µg |
| タバコの葉 | 60 µg |
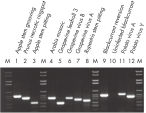
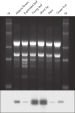
30~100 µlで溶出したRNAにはウイルスRNAが含まれています(図" ウイルスの検出")。多糖類等の夾雑物は全て除去されるため、溶出されたRNAはあらゆるダウンストリームアプリケーションにすぐに使用できます(図" ヒストンH4発現の組織特異性")。
図参照
原理
RNeasy Plant Mini Kitには、粘性の高い植物や菌類のライセートの微小遠心操作によるホモジナイゼーションおよびろ過用のQIAshredderカラムと、組み合わせて使用するRNA精製用のRNeasy Mini Spin Columnが付いています。グアニジンイソチオシアネート溶解と、シリカゲルメンブレンによる迅速な精製を組み合わせたRNeasyテクノロジーにより、トータルRNA精製を簡便に行なうことができます。RNeasy Kitで精製した高品質のRNAはほとんどDNAを含みません。微量のDNAに敏感なアプリケーションを行なう場合、DNase処理によってRNeasy操作中に残存するDNAを除去できます。
操作手順
図参照
アプリケーション
RNeasyテクノロジーで精製されたRNAは品質が高く、以下を含む幅広いアプリケーションに最適です。
- ノーザン、ドットおよびスロットブロット
- エンドポイントRT-PCR
- リアルタイム定量RT-PCR
- アレイ解析
- Poly A+ RNA 調製
裏付けデータと数値
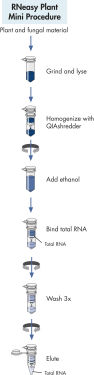
RNeasy Plant Mini procedure.
Specifications
| Features | Specifications |
|---|---|
| Applications | PCR, qPCR, real-time RT-PCR, microarray |
| Elution volume | 30–100 µl |
| Sample amount | 10–100 mg |
| Processing | Manual |
| Main sample type | Plant samples |
| Purification of total RNA, miRNA, poly A+ mRNA, DNA or protein | RNA |
| Format | Spin column |
| Technology | Silica technology |
| Yield | 25–60 µg |
| Time per run or per prep | 30 minutes |