QuantiFast Probe PCR Kits
配列特異的プローブを用いた高速なリアルタイムPCRおよび2ステップqRT-PCR
配列特異的プローブを用いた高速なリアルタイムPCRおよび2ステップqRT-PCR

Cat. No. / ID: 204256
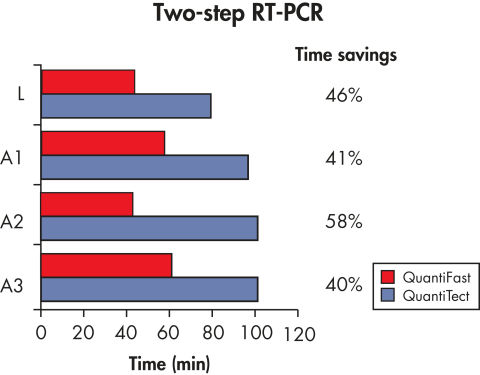
QuantiFast Probe PCR Kitsでは、他のリアルタイムPCRキットよりも卓越した感度の高い結果が得られます(表"iCycler iQを用いた高感度な2ステップRT-PCR"および図" 高感度な2ステップRT-PCR")。PCR時間が最大60%短縮され(図" PCR時間を顕著に短縮")、PCRの性能を妥協しなくてもPCR結果を速く得られます(図" 感度を損なわずにより迅速な結果を実現")。従って、サンプル処理数を大幅に増やしたり、他の実験者と1台のサイクラーを効率的に共有できます。
| 平均CT値 | ||
|---|---|---|
| ヒト白血球cDNA(ng) | QuantiFast Probe PCR +ROX Vial Kit | Supplier BVのキット |
| 100 | 29.13 | 31.13 |
| 10 | 32.50 | 34.43 |
| 1 | 36.15 | 40.00 |
| テンプレートを含まないコントロール | 40.00 | 40.00 |
QuantiFast Probe PCR Kitは幅広いダイナミックレンジにわたり正確な定量を実現します(図" 幅広いダイナミックレンジと高感度を実現")。テンプレートを最高107倍に希釈した溶液からでも正確な検出ができます(表"LightCycler 2.0を用いて107倍希釈したテンプレートを2ステップRT-PCRで検出"および図" 107倍希釈したテンプレートの検出")。
| 平均CT値 | ||
|---|---|---|
| Human leukocyte cDNA | QuantiFast Probe PCR +ROX Vial Kit | R 社Kit |
| 100 ng | 11.99 | 14.56 |
| 10 ng | 15.54 | 17.99 |
| 1 ng | 19.22 | 22.16 |
| 100 pg | 22.63 | 25.14 |
| 10 pg | 26.43 | 30.15 |
| 1 pg | 30.02 | 34.43 |
| 100 fg | 33.42 | 37.10 |
| テンプレートを含まないコントロール | 40 | 40.00 |
| PCR効率 | 90% | 80.1% |
QuantiFast Probe PCR Kitでは、標準的なサイクラー、高速サイクラーともに、広範なダイナミックレンジで非常に高感度な結果が迅速に得られます。本キットは、加水分解プローブの検出(TaqManあるいはその他のダブル標識プローブなどを含む)、FRETプローブなどの全てのタイプの配列特異的プローブを使用するためにデザインされています。新規添加剤のQ-Bondを含有する特別に開発された高速PCR用バッファーにより、変性、アニーリングおよびエクステンション時間も顕著に短縮されます(図" プライマーの高速なアニーリング")。このバッファーに入っているK+およびNH4+のイオン配合比により、プライマーとプローブが核酸テンプレートに効率的かつ安定してアニーリングし、高いPCR効率と感度を実現します(図" 特異的なプライマーアニーリング")。さらに、HotStarTaq Plus DNA Polymeraseは厳密なホットスタートを行なえるため、非特異的な産物の形成を抑えます。
| 成分 | 特長 | 利点 |
| HotStarTaq Plus DNA Polymerase | 95℃、3分間の活性化 | 室温での定量PCRのセットアップ |
| QuantiFast Probe PCR Buffer | NH4+/K+イオンの配合バランス | 特異性の高いプライマーのアニーリングで信頼性の高いPCR結果 |
| ユニークなQ-Bondを含む | PCR反応時間が短縮されるため迅速に結果が得られ、1日あたりの反応数を増やせる | |
| ROX色素† | Applied Biosystems社およびオプションのAgilent社の装置で蛍光シグナルを補正 | ROXが必要なサイクラーで正確な定量。他のリアルタイムサイクラーでの反応を妨害しない |
QuantiFast Probe PCR Kitは反応条件やサイクリング条件の至適化が不要で即使用可能なマスターミックスです。テンプレートDNA、プライマー、プローブをマスターミックスに添加し、ハンドブックに記載されているプロトコールに従うだけで、全てのリアルタイムサイクラーで迅速で正確な結果が得られます。マスターミックス中にROXパッシブリファレンス色素が入ったキットまたは入っていないキットをお求めいただけ、事実上全てのリアルタイムサイクラーでの使用が可能です(表参照)。ROX濃度が至適化されているため、コピー数が少ない場合の検出でも自動データ解析を行なうことができます。
| ROX色素 | Kit | 対応するサイクラー |
|---|---|---|
| マスターミックスに添加済み | QuantiFast Probe PCR Kit | Applied Biosystems 7500以外のApplied Biosystemsの全てのサイクラー |
| 別チューブで添付 | QuantiFast Probe PCR +ROX Vial Kit | Applied Biosystems 7500およびAgilent、Bio-Rad、Cepheid、QIAGEN、Eppendorf、Roche、その他のサイクラー |
2ステップリアルタイムRT-PCRで最適な結果を得るためには、QuantiTect Reverse Transcription Kitを用いてcDNAを合成することをお薦めします。本キットは、ゲノムDNAコンタミの除去を組み合わせたcDNA合成をわずか20分で行ないます。
QuantiFast Probe Assayは加水分解、プローブを用いた検出を利用したデザイン済みのゲノムワイドなアッセイです。QuantiFast Probe PCR Kitを使用して行なわれ、singleplex 2ステップqRT-PCRの結果が得られます。
QuantiFast Probe PCR Kitは、全てのリアルタイムサイクラーで、プローブを用いたcDNAターゲットの遺伝子発現解析および定量的gDNA解析に使用できます。これには、Applied BiosystemsおよびBio-Rad、Cepheid、Eppendorf、Roche、Agilent社のサイクラーを含みます。Rotor-Gene Qおよび他のRotor-Geneサイクラーを使用する場合は、これらの高速サイクリング用に特別に開発されたRotor-Gene Probe PCR Kitの使用をお勧めします。
| Features | Specifications |
|---|---|
| Applications | Probe-based, real-time PCR, two-step RT-PCR |
| Sample/target type | cDNA, DNA |
| Real-time or endpoint | Real-time |
| Reaction type | Real-time and two-step RT-PCR |
| Single or multiplex | Single |
| With or without ROX | Available with ROX in master mix and with ROX as separate vial |
| SYBR Green I or sequence-specific probes | Sequence-specific probes |
| Thermal cycler | All real-time cyclers (e.g. Roche LightCycler®, Corbett Rotor-Gene, Applied Biosystems) |