Omniscript RT Kit
反応あたり50 ng ~ 2 μg のRNA を用いた逆転写反応(エンドポイントPCR用)
反応あたり50 ng ~ 2 μg のRNA を用いた逆転写反応(エンドポイントPCR用)
Cat. No. / ID: 205113
Omniscript Reverse Transcriptase (RT) の高いRNAアフィニティーによりコピー数の少ない転写物からでさえも、高効率および高感度のRT-PCR結果が得られるので(図" 10コピー以上の高感度なRT-PCR")、温度あるいは反応条件の調整を行なわずに、複雑な二次構造を持つRNAでも、完全に読み取り、逆転写を実現します(図" 種々の逆転写酵素との比較")。
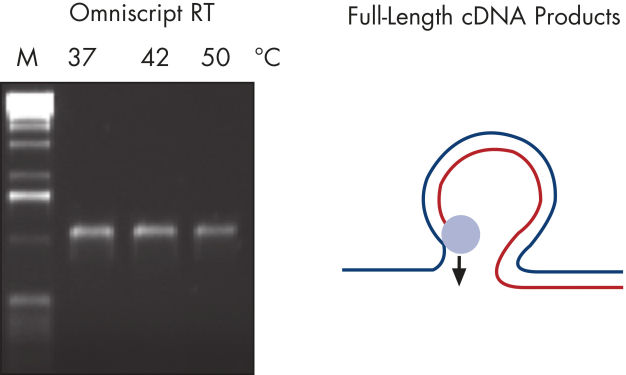
その他の逆転写酵素では、GC含有率の高いRNA領域は逆転写が止まったりRNAテンプレートから解離したりする原因であり、またRNAのループ構造部分をスキップしてしまいます(図" 完全長のRT-PCR産物 — B")。しかし、このような逆転写が困難なテンプレートも、QIAGENの逆転写酵素を使用すれば、逆転写が効率よく行なわれることが証明されています(図" 完全長のRT-PCR産物 — A")。Omniscript RT Kitは、至適化なしで事実上問題なく逆転写が可能なキットです。
RNAに対して高いアフィニティーを有するOmniscript Reverse Transcriptase(RT)は、どのようなテンプレートでも効率的で高感度の逆転写が可能であり、高収量のcDNAが得られます。dNTPsと至適化済みの反応バッファーが同梱されているため即使用可能であり、RNAに対するOmniscript RTの高いアフィニティーと相まって、高GC含量または複雑な二次構造のテンプレートの読み取りが実現しています。プライマーミックスは同梱されていなのでご注意ください。
Omniscript RTは、各反応あたり50 ngから2 µg のRNA量を用いた、あらゆる逆転写用に特別にデザインされています。多くのウイルスRNA調製にはキャリアRNAが存在するので、Omniscript RTは通常ウイルスRNAに最適な酵素です。比較実験においても、Omniscript RTは、幅広いRNAのスタート量で他の逆転写酵素より一定した優れた性能を示します。
Omniscript Kitsのロット間の再現性は、QIAGENの厳しい品質管理により保証されています。全てのOmniscript RT Kitsに添付されている至適化済みのBuffer RT、dNTPs、水はRNaseフリーの保証付きで、Omniscript RTの各ロットは、RT-PCRの再現性に関する多数の品質管理テストを受けています。
Omniscript Reverse Transcriptaseは以下のようなアプリケーションに最適です。
| Features | Specifications |
|---|---|
| Applications | RT-PCR, qRT-PCR, primer-extension, RACE analysis |
| Real-time or endpoint | Endpoint |
| Mastermix | No |
| Enzyme activity | Reverse transcription |
| Single or multiplex | Single |
| With/without hotstart | Without hotstart |
| Reaction type | Reverse transcription |
| Sample/target type | RNA template |