✓ オンライン注文による24時間年中無休の自動処理システム
✓ 知識豊富で専門的な製品&テクニカルサポート
✓ 迅速で信頼性の高い(再)注文
RNeasy Lipid Tissue Mini Kit (50)
Cat. No. / ID: 74804
50 RNeasy Mini Spin Columns, Collection Tubes (1.5 ml and 2 ml), QIAzol Lysis Reagent, RNase-free Reagents and Buffers
RNeasy Lipid Tissue Mini Kitは分子生物学的アプリケーション用であり、疾病の診断、予防、あるいは治療に使用することはできません。
✓ オンライン注文による24時間年中無休の自動処理システム
✓ 知識豊富で専門的な製品&テクニカルサポート
✓ 迅速で信頼性の高い(再)注文
特徴
- 脂肪性組織およびその他の組織に最適な溶解条件
- フェノールのコンタミがない高収量のトータルRNA
- あらゆるダウンストリームアプリケーションに対応する高品質RNA
製品詳細
RNeasy Lipid Tissue Mini Kitには、脂肪性組織およびその他のタイプの組織サンプルを溶解するQIAzol Lysis Reagentと、100 µgまでの高品質RNA精製のためのRNeasy Spin Columnが含まれています。本キットはQIAcubeを用いて自動化可能です。組織サンプルはRNAlater RNA Stabilization Reagent(非脂肪性組織のみ)あるいはAllprotect Tissue Reagentで簡便に安定化され、TissueRuptorあるいはTissueLyserシステムで効率的に破砕されます。より多量のサンプルにはRNeasy Lipid Tissue Midi Kit(スピンカラム結合容量はRNA 1 mg)をご使用ください。
パフォーマンス
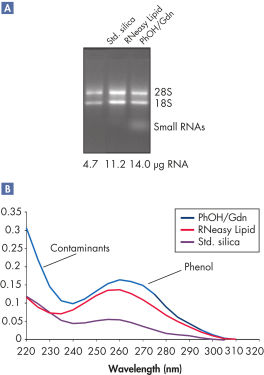
RNeasy Lipid Tissue Kitは脂肪の多い組織での使用に至適化されており、代表的な脂肪性組織である脳および脂肪組織から、10mgあたりそれぞれ10ugおよび2.4ugのRNAを精製できます。RNeasy Lipid Tissue kitにより、高品質なトータルRNAを簡単かつ効率的に精製することができ、フェノールのキャリーオーバーもありません(図" フェノールのキャリーオーバーのない高収量RNA")。本キットを用いて脂肪性組織から精製したRNAは、リアルタイムRT-PCRなどのダウンストリームアプリケーションに適しています(図" 高品質RNAのリアルタイム解析")。
図参照
原理
RNeasy Lipid Tissue Kitは脳や脂肪組織のような脂肪性組織での使用に至適化されています。簡便なRNeasy Lipid Tissueプロトコールは、フェノール/グアニジンベースの溶解法を実績のあるRNeasy調製法と統合し、高品質トータルRNAの高い収量での精製を可能にしています。有機溶媒抽出とカオトロピック塩による組織破砕の組み合わせにより、QIAzol Lysis Reagentの高い溶解効率を実現します。200 塩基以上のその他のRNA のみを精製するので、精製物は全細胞性RNAの15~20%を形成する5S rRNA、tRNA、その他の低分子RNAを含みません。
操作手順
10~100 mgの組織サンプルをQIAzol Lysis Reagent中でホモジナイズします。クロロホルムを添加した後、遠心操作によりホモジネートを水層と有機溶媒層に分離します。上部の水層を抽出し、エタノールを添加して適切な結合条件にします。サンプルをRNeasy Spin Columnにアプライすると、トータルRNA(最大100 µg)がメンブレンに結合し、フェノールおよびその他の夾雑物は効率的に除去されます。高品質なトータルRNAを30~100 µlのRNaseフリー水で溶出します(図" RNeasy Lipid Tissue Mini操作手順")。
図参照
アプリケーション
RNeasy Lipid Tissue Kitで精製した高品質RNAは、アレイ解析やリアルタイムRT-PCRを含むあらゆるダウンストリームアプリケーションに最適です。
裏付けデータと数値
High yields of RNA without phenol carryover.
RNA was isolated from 10 mg rat brain tissue using the RNeasy Lipid Tissue Mini Kit (RNeasy Lipid), a standard silica-gel-membrane procedure (Std. silica), or a phenol/guanidine-based reagent (PhOH/Gdn), following supplier's instruction. [A] Formaldehyde agarose gel analysis shows high yields of RNA using the RNeasy Lipid Tissue Mini Kit. Using the RNeasy and other silica-based methods, small RNAs (such as 5.8S rRNA, 5S rRNA, and tRNAs) are selectively excluded. [B] Absorbance spectrum shows contaminants and phenol when using the phenol/guanidine-based reagent.
Specifications
| Features | Specifications |
|---|---|
| Applications | NGS, PCR, real-time PCR, microarray |
| Sample amount | 10–100 mg |
| Yield | 2.4–10 mg |
| Technology | Silica technology |
| Processing | Manual |
| Purification of total RNA, miRNA, poly A+ mRNA, DNA or protein | RNA |
| Elution volume | 30–100 µl |
| Main sample type | Fatty tissue samples |
| Time per run or per prep | 45 minutes |
| Format | Spin column |
リソース
MSDS (1)
パンフレット (3)
User-Developed Protocols (4)
キットハンドブック (1)
クイックスタートプロトコール (1)
Safety Data Sheets (1)
Certificates of Analysis (1)
Publications
Lipopolysaccharide activates an innate immune system response in human adipose tissue in obesity and type 2 diabetes.
Am J Physiol Endocrinol Metab; 2006; 292 (3):E740-7 2006 Nov 7 PMID:17090751
Chronic ethanol feeding to rats decreases adiponectin secretion by subcutaneous adipocytes.
Am J Physiol Endocrinol Metab; 2006; 292 (2):E621-8 2006 Oct 17 PMID:17047161
Targeting an E2F site in the mouse genome prevents promoter silencing in quiescent and post-mitotic cells.
Oncogene; 2006; 26 (19):2727-35 2006 Oct 30 PMID:17072340
Paternally biased X inactivation in mouse neonatal brain.
Genome Biol; 2010; 11 (7):R79 2010 Jul 27 PMID:20663224
A study of alternative splicing in the pig.
BMC Res Notes; 2010; 3 :123 2010 May 5 PMID:20444244
FAQ
Which kit should I use for RNA isolation from Cartilage?
I accidentally stored Buffer RDD of the RNase-Free DNase Set at°C. Will it still function?
What is the smallest sample size that can be used with RNeasy Mini Kits?
Is it possible to isolate both RNA and recombinant 6xHis-tagged protein from the same sample?
Can the AllPrep DNA/RNA/Protein Mini Kit be used with fibrous or lipid tissues?
Do you have a kit for RNA isolation from any kind of sample type?
What is the composition of Buffer RLT?
How can I avoid little or no RNA yields when using an RNeasy Kit?
When using QIAzol Lysis Reagent for RNA extraction, I see a pinkish colored aqueous phase! Is it OK?
Do you have a protocol for the isolation of RNA from bacterial cultures using RNAprotect Bacteria Reagent and QIAzol Lysis Reagent?
How can I check the integrity of RNA purified using RNeasy Kits?
What is the maximum binding capacity of RNeasy spin columns?
Do you have a protocol for purification of total RNA from plant latex?
How can I check for purity of RNA isolated using RNeasy Kits?
Do you have a protocol for the isolation of genomic DNA and/or proteins from fatty tissue treated with QIAzol?
Do you have a protocol for purification of total RNA from fatty tissues using QIAzol Lysis Reagent and MaXtract High Density?
Are RNeasy spin columns sold separately?
I ran my RNA out on an agarose gel and can see lots of bands similar to a ladder. Why?
Which chloroform should I use for the RNeasy Lipid Tissue Kit?
What is the maximum volume of RNA in solution that can be used with the QuantiTect Whole Transcriptome Kit?
What is the difference between disruption and homogenization in the RNeasy System?
How is the RNeasy Lipid Tissue Mini Kit different from the RNeasy Mini Kit?
Can the RNase-Free DNase Set be used for DNase digestions of RNA in solution?
Do you have a protocol for purification of total RNA from plant tissue with the RNeasy Lipid Tissue Mini Kit?
Can the MaXtract High Density Tube be used with QIAzol to isolate RNA?
How should RNeasy Kits be stored and how long are they stable?
Which kit should be used to extract RNA from adipose tissue, brain, and other fatty animal tissues?
Why are samples centrifuged at 4°C after the addition of chloroform when using RNeasy Lipid Tissue Kits?
Can I buy the RNeasy Mini columns, RNeasy MinElute columns, RNeasy Midi columns or the RNeasy Maxi columns separately?
What can be used as an alternative to the A260 measurement for quantification of small amounts of RNA and DNA?
How do you ensure that RNeasy buffers are RNase-free?
Do I need to use RNase inhibitors with the RNeasy Kits?
Have you observed co-amplification of genomic DNA from RNA templates used in the QuantiTect Whole Transcriptome Procedure?
What are the effects of low A260/A230 ratios in RNA preparations on downstream applications?
What has to be done to an RNA sample before loading it onto an Agilent Bioanalyzer?
How can I ensure complete genomic DNA removal when using the RNase-Free DNase Set?
Can I homogenize samples in QIAzol using the QIAshredder?
What happens if I spin my lysate on the RNeasy Spin Columns at maximum speed?
Why does my isolated RNA have a low OD 260/280 ratio?
How do I safely inactivate biohazardous flow-through material?
What is the composition of Buffer RPE?
What is the composition of Buffer RW1?
Can I use the RNeasy Mini Kit or RNeasy 96 Kit with fewer than 100 cells?
How should I quantify RNA isolated with RNeasy Kits?