qBiomarker Somatic Mutation PCR Arrays
体細胞DNA変異の状態を迅速かつ正確にプロファイリングできる遺伝子パネル
体細胞DNA変異の状態を迅速かつ正確にプロファイリングできる遺伝子パネル
Cat. No. / ID: 337021
qBiomarker Somatic Mutation PCR Arrayは翻訳研究ツールで、生物学的パスウェイあるいは疾病に関連する重要な遺伝子の体細胞変異状態のプロファイリングを迅速かつ正確に行ないます。変異は、臨床または機能的関連性および出現頻度に基づいて、広範な体細胞突然変異のデータベース(例;COSMIC)と査読済み科学文献から選択されています。
qBiomarker Somatic Mutation PCR Arrayはホルマリン固定パラフィン包埋(FFPE)組織でも高い感度を示します(図 “ 高い感度” および “ FFPEサンプルを用いた場合のqBiomarker Somatic Mutation PCR Array感度”)。
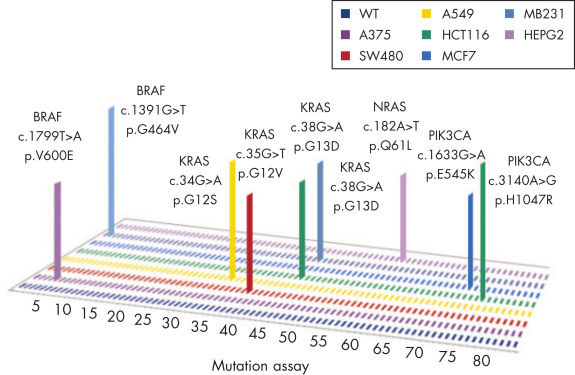
qBiomarker Somatic Mutation PCR Arrayは、毒性研究、創薬、がん研究において重要となる、細胞株または研究サンプル中の変異検出に有用です(図 “ 一般的ながん細胞株の体細胞突然変異状態のプロファイリング”)。
DNAテンプレートと即使用可能なPCRマスターミックスを、同一プレートの各ウェルに等量分注し、リアルタイムPCRサイクリングプリグラムを起動します。 qBiomarker Somatic Mutation PCR ArrayはABI、Bio-Rad、Eppendorf、Roche、Stratagene社の装置で使用可能です。
96ウェルおよび384ウェルプレートでの入手が可能なqBiomarker Somatic Mutation PCR Arrayは、疾病状態やパスウェイに関連する変異検出に加えて、標準化のための遺伝子コピー数コントロール用に使用します。各qBiomarker Somatic Mutation PCR Arrayにはまた、一般的なPCRパフォーマンスをチェックするためのコントロールが含まれています。
エクセルベースのデータ解析テンプレートを用いてデータ解析を行なえます。データ解析は∆∆CT 法あるいは平均 ∆CT 法をベースにしています。
アレイは様々なフォーマットでお届けし、すべてマスターミックスが入っています。
qBiomarker Somatic Mutation PCR Arrayは、パスウェイあるいは疾病に特異的な遺伝子セットおよびダウンストリームでシグナル伝達に関与する遺伝子の変異を迅速かつ正確にプロファイリングできます。