QIAGEN Plasmid Kits
10 mgまでのトランスフェクショングレードのプラスミドおよびコスミドDNA精製用
10 mgまでのトランスフェクショングレードのプラスミドおよびコスミドDNA精製用
✓ オンライン注文による24時間年中無休の自動処理システム
✓ 知識豊富で専門的な製品&テクニカルサポート
✓ 迅速で信頼性の高い(再)注文
Cat. No. / ID: 12123
✓ オンライン注文による24時間年中無休の自動処理システム
✓ 知識豊富で専門的な製品&テクニカルサポート
✓ 迅速で信頼性の高い(再)注文
QIAGEN Plasmid Kits はオープンカラム方式の陰イオン交換チップを用いてトランスフェクショングレードのプラスミドDNA 精製を実現します。ライセート清澄化およびイソプロパノール沈殿は遠心操作により行ないます。
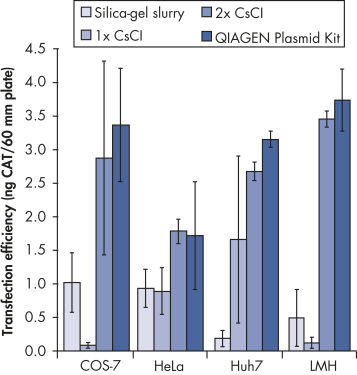
QIAGEN-tip 中の非常にユニークな陰イオン交換樹脂は核酸精製を目的として開発されました。本製品の優れた核酸分離能力により、CsCl 密度勾配遠心分離法を2回行なって得たDNAの純度に匹敵、あるいはそれ以上の純度のDNAが調製されます。充填済みQIAGEN-tipsはオープンカラムで操作し、乾燥することはなく、プラスミド調製に必要なマニュアルでの作業時間を短縮できます。全てのQIAGENプラスミド精製システムでは、ユーザーおよび環境への影響が最小限となるように、フェノール、クロロホルム、臭化エチジウム、CsCl等の有害な試薬を一切使用していません。
特徴 | Plasmid Giga Kit | Plasmid Mega Kit | Plasmid Maxi Kit | Plasmid Midi Kit | Plasmid Mini Kit |
| Applications | Transfection, cloning, sequencing, capillary sequencing, etc. | Transfection, cloning, sequencing, capillary sequencing, etc. | Transfection, cloning, sequencing, capillary sequencing, etc. | Transfection, cloning, sequencing, capillary sequencing, etc. | Transfection, cloning, sequencing, capillary sequencing, etc. |
| Culture volume/starting material | 2.5–5 liters culture volume | 500 ml – 2.5 liters culture volume | 100–500 ml culture volume | 25–100 ml culture volume | 3–10 ml culture volume |
| Elution volume | Variable | Variable | Variable | Variable | Variable |
| Plasmid type | High-copy, low-copy, cosmid DNA | High-copy, low-copy, cosmid DNA | High-copy, low-copy, cosmid DNA | High-copy, low-copy, cosmid DNA | High-copy, low-copy, cosmid DNA |
| Processing | Manual (centrifugation) | Manual (centrifugation) | Manual (centrifugation) | Manual (centrifugation) | Manual (centrifugation) |
| Sample per run | 1 sample per run | 1 sample per run | 1 sample per run | 1 sample per run | 1 sample per run |
| Technology | Anion-exchange technology | Anion-exchange technology | Anion-exchange technology | Anion-exchange technology | Anion-exchange technology |
| Time per run | 320 min | 220 min | 160 min | 150 min | 80 min |
| Yield | <10 mg | <2.5 mg | <500 µg | up to 100 µg | <20 µg |
QIAGEN Plasmid Kitsを使用して、遠心操作でバクテリアライセートを清澄化します。清澄化したライセートを陰イオン交換チップ上にアプライすると、適切な低塩あるいはpH条件でプラスミドDNAが選択的に結合します。RNA、タンパク質、二次代謝物、その他の低分子量不純物が、中濃度の塩による洗浄で取り除かれ、高純度プラスミドDNAが高塩濃度のバッファーで溶出されます(フローチャート" QIAGEN Plasmid Kits 操作の比較"参照)。イソプロパノール沈殿によりDNAが濃縮・脱塩され、遠心操作により回収されます。
QIAGEN Plasmid Kits を用いて精製したプラスミドDNAは、以下のようなアプリケーションに最適です。
| Features | Specifications |
|---|---|
| Technology | Anion-exchange technology |
| Culture volume/starting material | 3 ml–5 liters culture volume |
| Yield | <20 µg to <10 mg |
| Processing | Manual (gravity flow) |
| Samples per run (throughput) | 1 sample per run |
| Time per run or prep per run | 80–320 min |
| Applications | Transfection, cloning, sequencing, capillary sequencing etc. |
| Plasmid type | High-copy, low-copy, cosmid DNA |