✓ 24/7 automatic processing of online orders
✓ Knowledgeable and professional Product & Technical Support
✓ Fast and reliable (re)-ordering
QIAcuity High Multiplex Probe PCR Kit (1mL)
Cat. No. / ID: 250133
✓ 24/7 automatic processing of online orders
✓ Knowledgeable and professional Product & Technical Support
✓ Fast and reliable (re)-ordering
Features
- For multiplexing beyond 5-plex including amplitude multiplexing of up to 12-plex
- 4x concentrated master mix that enables loading of more sample
- Optimized for microfluidic use in QIAcuity Nanoplates
- REACH-compliant kit
Product Details
The QIAcuity High Multiplex Probe PCR Kit contains a 4x concentrated, ready-to-use master mix optimized for microfluidic use in the QIAcuity Nanoplates. The kit enhances the specificity and efficiency of probe-based digital PCR to provide accurate singleplex, or up to 12-plex, analysis. You can set up 8-plex reactions by using six QIAcuity channels (one assay/channel) plus two Long Stokes-Shift (LSS) dyes. For analysis of up to 12 targets, you can perform amplitude multiplexing by mixing two different assays per channel.
The QIAcuity High Multiplex Probe Master Mix is optimized to increase specificity for accurate quantification of gDNA or cDNA on the QIAcuity dPCR instruments.
The kit works in conjunction with the QIAcuity Digital PCR System and the QIAcuity Nanoplates.
Would you like to find out more about the product from one of our dPCR specialists? Sign in here, and we will get in touch with you shortly.
Performance
The QIAcuity digital PCR platform overcomes the challenges of high multiplexed PCR in part thanks to the custom crosstalk matrix in QIAcuity Software 3.0. This new software feature compensates for optical crosstalk, or signal overlap, between neighboring channels. Together with the specialized chemistry of the QIAcuity High Multiplex Probe PCR Kit, users can detect targets in up to eight channels: six standard optical channels and two hybrid channels that make use of hydrolysis probes with Long Stokes-Shift (LSS) dyes ( Figure 1).
QIAcuity Software 3.1 and the QIAcuity High Multiplex Probe PCR Kit allow users to detect up to 12 targets in six channels ( Figure 2 and Figure 3).
See figures
Principle
Thanks to a novel, antibody-mediated, hot-start mechanism, the QIAcuity High Multiplex Probe PCR Kit delivers singleplex or multiplex cDNA or gDNA analysis with the highest specificity. At low temperatures, the QuantiNova DNA Polymerase is kept in an inactive state by the QuantiNova Antibody and a novel additive, QuantiNova Guard, that stabilizes the complex. This improves the stringency of the hot-start and prevents extension of nonspecifically annealed primers and primer–dimers. Within 2 minutes of raising the temperature to 95°C, the QuantiNova Antibody and QuantiNova Guard are denatured, and the QuantiNova DNA Polymerase is activated, enabling PCR amplification.
The principle of the dPCR reaction in the nanoplates is described here.
Procedure
The QIAcuity High Multiplex Probe Kit contains a ready-to-use 4x PCR master mix, eliminating the need to optimize reaction and cycling conditions. Simply add the template DNA and primer-probe sets to the master mix and follow the procedure outlined in the Quick-Start Protocol to get fast and reliable results on the QIAcuity.
The system integrates partitioning, thermocycling and imaging into a single fully automated instrument that takes users from the sample to result in under 2 hours. Analyze your results on the Software Suite, where you can find the concentration of your target sequence in copies per microliter and obtain data on positive samples or NTC for quality control purposes. This analysis can be extended to remote computers within the same local area network (LAN) for added convenience.
Applications
The QIAcuity High Multiplex Probe PCR Kit, in combination with the QIAcuity Digital PCR System and the QIAcuity Nanoplates, enables quantitative analysis of cDNA targets and gDNA for use in applications, including:
- Rare mutation detection
- Copy number variation analysis
- Microbial research including wastewater monitoring
- Pathogen detection
- Genotyping
- Gene expression analysis
- miRNA research
Supporting data and figures
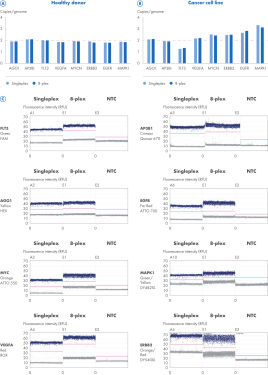
Detect more CNV targets with great confidence in a single reaction.
Eight-plex reactions were assembled to compare the copy number of six genes of interest (GOIs) (FLT3, VEGFA, MYCN, ERBB2, EGFR, MAPK1) between a healthy human donor and the U-2 OS sarcoma cell line. The average copy number of the reference genes AGO1 and AP3B1 was used for normalization. All eight gene targets were also quantified in singleplex for comparison. In both singleplex and 8-plex reactions, the copy number of the six GOIs and the two reference genes in the healthy donor was around 2, as expected. In contrast, the results from both the singleplex and 8-plex reactions revealed genome instability in the sarcoma cell line, with the GOI copy numbers varying from 1.25 to 3.3. Even the reference genes displayed slight instability.