QuantiTect Probe RT-PCR Kit (200)
Cat. No. / ID: 204443
特徴
- 低コピーターゲットを高感度で検出
- 配列特異的プローブまたはSYBR Greenを用いるqRT-PCR
- テンプレートの複数のログにわたる正確な定量
- 反応条件やサイクリング条件の最適化が不要
- 同一チューブ内で参照遺伝子と最大3つの標的を検出
製品詳細
QuantiTect RT-PCR Kitは、配列特異的プローブまたはSYBR Green I検出を用いるリアルタイム1ステップPCRによるRNAたーげっとの高感度定量を可能にします。また、このキットは、マルチプレックスのリアルタイム1ステップRT-PCRにより、1つのチューブで最大5つのRNAターゲットの信頼性の高い定量を可能にします。すぐに使用できるマスターミックスにホットスタートと独自のPCRバッファーシステムを組み合わせることにより、最適化を必要とせず、どのリアルタイムサイクラーでも高感度qRT-PCRを保証します。dNTPミックスにはdUTPが含まれており、オプションでUNGによる処理が可能です。QuantiTect 1ステップRT-PCRキットではマスターミックスが2–8°Cで保存でき、とても便利です。
配列特異的プローブを使用するマルチプレックスRT-PCRには、蛍光正規化にROX色素を必要とするサイクラー用のQuantiTect Multiplex RT-PCR Kitと、その他すべてのサイクラー用のQuantiTect Multiplex RT-PCR NoROX Kitの2つのキット形式があります。QuantiTect SYBR Green RT-PCR Kitには、広範囲のRNAテンプレート量に対して効率的かつ高感度な逆転写ができるように最適化されたRTミックスが付属しています。
パフォーマンス
QuantiTect1ステップRT-PCRキットに含まれるHotStarTaq DNA Polymeraseは、他のポリメラーゼに比べ、最も厳密なホットスタートを提供することにより、PCR反応の特異性を高めます。
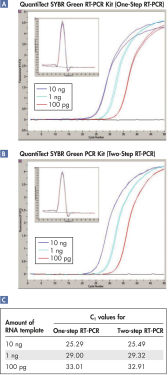
QuantiTect SYBR Green RT-PCR Kitは、独自のブレンドの逆転写酵素を含み、広範囲な量のRNAテンプレートにわたり効率的かつ高感度なcDNA合成を実現します。また、広い直線範囲にわたり特異的定量を可能にします(図「 1ステップRT-PCRと2ステップRT-PCRキットは同等の結果を示す」参照)。
QuantiTect Probe RT-PCR Kitは、プライマーとプローブのPCRテンプレートへの非常に特異的なアニーリングを促進する独自のRT-PCRバッファーを備えています。HotStarTaq DNA PolymeraseとRT-PCRバッファーの独自の組成により、QuantiTect Probe RT-PCR Kitは低コピーRNAターゲットの高感度定量と、広い直線範囲にわたる正確な定量を可能にします(図「 高い感度と効率、広いダイナミックレンジ」参照)。
逆転写とPCRを同じチューブで連続して行っても感度は損なわれません。これは、リアルタイム2ステップRT-PCRで達成されるCT値と同じCT値であることによって実証されています(図「 2ステップRT-PCRと同等の性能を持つ1ステップRT-PCR – A」および「 2ステップRT-PCRと同等の性能を持つ1ステップRT-PCR – B」、および表“2ステップRT-PCRと同等の性能を示す1ステップRT-PCR”参照)。
2ステップRT-PCRと同じ性能を示す1ステップRT-PCR
| cDNA/RNAの量 |
平均Ct値 (2ステップRT-PCR) |
平均Ct 値 (1ステップRT-PCR) |
|---|---|---|
| 100 ng | 24.66 | 24.66 |
| 10 ng | 28.15 | 27.84 |
| 1 ng | 31.42 | 31.49 |
| 0.1 ng | 34.80 | 34.56 |
| 0.01 ng | 37.78 | 37.66 |
QuantiTect Multiplex RT-PCR Kitを使用すると、マルチプレックスqRT-PCRでは、わずか10コピーのターゲット配列(図 「 デュプレックスPCRではターゲットRNAを10コピーまで検出 」参照)を高感度に検出できます。遺伝子発現の正確な相対定量は、ターゲット遺伝子と内因性コントロール遺伝子の両方の発現を同じウェルまたはチューブで定量することで達成されます。QuantiTect Multiplex RT-PCR Kitを用いると、マルチプレックス反応におけるターゲットのCT値は、ターゲットを別々の反応で増幅するコントロール実験で得られるCT値と同等です(図「 トリプレックスPCRとシングルプレックスPCRの増幅は同じ」参照)。これは、同じマルチプレックス反応中の異なるターゲットが、互いに影を与え合うことなく効率的かつ高感度に増幅されることを示しています。
図参照
原理
QuantiTect RT-PCR Kitには、SYBR Green Iまたは配列特異的プローブを用いてRNAターゲットを高特異的かつ高感度にリアルタイム定量することができるように最適化された、すぐに使えるマスターミックスが含まれています。QuantiTect SYBR Green RT-PCRマスターミックスに含まれる蛍光色素SYBR Green Iは、数多くのさまざまなターゲットの分析を可能にし、ターゲット特異的標識プローブを合成する必要はありません(表“2x QuantiTect SYBR Green RT-PCR Kitのコンポーネント”参照)。
2x QuantiTect SYBR Green RT-PCR Kitのコンポーネント
| コンポーネント | 特徴 | メリット |
|---|---|---|
| HotStarTaq DNA Polymerase | 95ºC 15分で活性化 | 室温でqPCR反応をセットアップ |
| QuantiTect SYBR Green RT-PCR Buffer | NH4+イオンとK+イオンのバランスの取れた組み合わせ | 特異的なプライマーアニーリングにより信頼性の高いPCR結果を保証 |
| dNTPミックス | dTTPを部分的に置換し、オプションで反応のUNG処理を可能にするdUTPを含む | オプションのUNG処理により、PCR産物のキャリーオーバーによるコンタミネーションを排除 |
| SYBR Green I色素 | 二本鎖DNAと結合すると強い蛍光シグナルを発する | 高感度定量 |
| ROX色素 | Applied BiosystemsおよびAgilentの装置(オプション)での蛍光シグナルの正規化用 | ROX色素を必要とするサイクラーでの正確な定量。他のリアルタイムサイクラーの反応に干渉しない |
| OmniscriptおよびSensiscript逆転写酵素 | RNAに高い親和性を持つ酵素の特別ブレンド | RNAは複雑な二次構造を介しても転写可能 |
QuantiTect Probe RT-PCR Kitには、配列特異的プローブを用いてRNAターゲットを高特異的かつ高感度にリアルタイム定量することができるように最適化された、すぐに使えるマスターミックスが含まれています(表“2x QuantiTect Probe RT-PCR Kitのコンポーネント”参照)。このキットは、加水分解プローブ(TaqMan®やその他の二重ターゲットプローブなど)、FRETプローブ、Molecular Beaconなど、あらゆるタイプの配列特異的プローブに使用できるように設計されています。
2x QuantiTect Probe RT-PCR Kitのコンポーネント
| コンポーネント | 特徴 | メリット |
|---|---|---|
| HotStarTaq DNA Polymerase | 95ºC 15分で活性化 | 室温でqPCR反応をセットアップ |
| QuantiTect Probe RT-PCR Buffer | NH4+イオンとK+イオンのバランスの取れた組み合わせ | 特異的なプライマーアニーリングにより信頼性の高いPCR結果を保証 |
| dNTPミックス | dTTPを部分的に置換し、オプションで反応のUNG処理を可能にするdUTPを含む | オプションのUNG処理により、PCR産物のキャリーオーバーによるコンタミネーションを排除 |
| SYBR Green I色素 | 二本鎖DNAと結合すると強い蛍光シグナルを発する | 高感度定量 |
| ROX色素 | Applied BiosystemsおよびAgilentの装置(オプション)での蛍光シグナルの正規化用 | ROX色素を必要とするサイクラーでの正確な定量。他のリアルタイムサイクラーの反応に干渉しない |
| OmniscriptおよびSensiscript逆転写酵素 | RNAに高い親和性を持つ酵素の特別ブレンド | RNAは複雑な二次構造を介しても転写可能 |
遺伝子発現の正確な相対定量は、ターゲット遺伝子と内因性コントロール遺伝子の両方の発現を同じウェルまたはチューブで定量することで達成されます。QuantiTect Multiplex RT-PCR Kitの最適化されたマスターミックスは、マルチプレックス反応におけるPCR産物が、対応する単一増幅反応におけるPCR産物と同じ効率と感度で増幅されることを保証します(表“2x QuantiTect Multiplex RT-PCR Kitのコンポーネント”参照)。このキットでは、わずか10コピーのターゲット遺伝子を検出できます。
2x QuantiTect Multiplex RT-PCR Kitのコンポーネント
| コンポーネント | 特徴 | メリット |
|---|---|---|
| HotStarTaq DNA Polymerase | 95ºC 15分で活性化 | 室温でqPCR反応をセットアップ |
| QuantiTect Multiplex RT-PCR Buffer | NH4+イオンとK+イオンのバランスの取れた組み合わせ | 特異的なプライマーアニーリングにより信頼性の高いPCR結果を保証 |
| 合成Factor MP | 同じチューブで最大4つの遺伝子を高い信頼性でマルチプレックス分析 | |
| dNTPミックス | dTTPを部分的に置換し、オプションで反応のUNG処理を可能にするdUTPを含む | オプションのUNG処理により、PCR産物のキャリーオーバーによるコンタミネーションを排除 |
| ROX色素* | Applied BiosystemsおよびAgilentの装置(オプション)での蛍光シグナルの正規化用 | ROX色素を必要とするサイクラーでの正確な定量。他のリアルタイムサイクラーの反応に干渉しない |
| OmniscriptおよびSensiscript逆転写酵素 | RNAに高い親和性を持つ酵素の特別ブレンド | RNAは複雑な二次構造を介しても転写可能 |
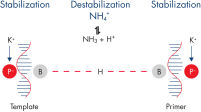
QuantiTect RT-PCR KitのPCRバッファーに含まれるK+イオンとNH4+イオンのバランスの取れた組み合わせ – および、QuantiTect Multiplex RT-PCRバッファー中の独自の合成Factor MP – は、特異的プライマーアニーリングを促進し、高いRT-PCR特異性と感度を可能にします(図「 特異的プライマーアニーリング」参照)。さらに、逆転写酵素の最適化された混合が、広範囲のRNAテンプレート量からのcDNA合成を可能にします。HotStarTaq DNA Polymeraseは厳密なホットスタートを提供し、非特異的産物の形成を防止します。
QuantiTect RT-PCRマスターミックスにはdUTPも含まれているので、PCR開始前にウラシル-N-グリコシラーゼ(UNG)で前処理することができるため、確実にPCR産物のコンタミネーションがその後のPCR反応に影響を与えないようにすることができます。
図参照
操作手順
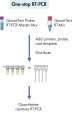
QuantiTect RT-PCR Kitは、面倒で時間がかかる可能性のある反応条件の最適化を必要としません。すぐに使用できるQuantiTect SYBR Green RT-PCRマスターミックスにプライマーとテンプレートを加えて、 – またはすぐに使用できるQuantiTect Probe RT-PCRマスターミックスにプライマー、プローブ、テンプレートを加えて – 反応を開始させるだけです(フローチャート「 SYBR Greenを使用する1ステップRT-PCR」と 「“ 配列特異的プローブを使用するRT-PCR」を参照)。ハンドブックのプロトコールに従って、どのリアルタイムサイクラーでも迅速に信頼性の高い結果が得られます。必要に応じて、反応をウラシル-N-グリコシラーゼ(UNG)で前処理して、前の反応からのPCR産物のキャリーオーバーを除去することができます。
QuantiTect SYBR Green RT-PCR KitをQuantiTect Primer Assaysと組み合わせて使用すると、遺伝子発現分析で非常に特異性の高い結果が保証されます。これらは、ヒト、マウス、ラット、その他多くの種の転写産物を検出するための、ゲノムワイドなバイオインフォマティクス的に検証されたプライマーセットです。QuantiTect Primer Assaysは、GeneGlobeで簡単に注文できます。
QuantiTect Multiplex RT-PCR Kitのハンドブックには、利用可能なすべてのリアルタイムサイクラーで使用できる単一プロトコールが記載されており、推奨色素もリストされています。キットは、マスターミックス中にROXパッシブレファレンス色素を含むものと含まないものがあり、実質的にどのリアルタイムサイクラーでも使用できます(表“適切なQuantiTect Multiplex RT-PCR Kitの選択”参照)。ROX濃度が最適化されているため、低コピー数であっても自動データ分析によって検出が可能です。
適切なQuantiTect Multiplex RT-PCR Kitの選択
| ROX色素 | キット | 互換性のあるサイクラー |
|---|---|---|
| マスターミックスに含まれる | QuantiTect Multiplex RT-PCR Kit | Applied Biosystemsのサイクラー |
| マスターミックスに含まれない | QuantiTect Multiplex RT-PCR NR Kit | Rotor-Geneサイクラー、およびBio-Rad、Cepheid、Eppendorf、Roche、Agilent、その他のサプライヤーのサイクラー |
図参照
アプリケーション
QuantiTect RT-PCR Kitは、あらゆるリアルタイムサイクラーでのRNAターゲットの遺伝子発現分析に使用できます。これには、Applied Biosystems、Bio-Rad、Cepheid、Eppendorf、Roche、Agilentの装置が含まれています。Rotor-Gene Qおよびその他のRotor-Geneサイクラーでは、これら装置での高速サイクリング用に特別に開発されたRotor-Gene Probe RT-PCR Kit、Rotor-Gene Multiplex RT-PCR Kit、またはRotor-Gene SYBR Green RT-PCR Kitの使用をお勧めします。
| 特徴 | QuantiTect SYBR Green RT-PCR Kit | QuantiTect Probe RT- PCR Kit | QuantiTect Multiplex RT-PCR Kit |
|---|---|---|---|
| アプリケーション | RNAターゲットのリアルタイム定量 | RNAターゲットのリアルタイム定量 | マルチプレックス形式でのRNAターゲットのリアルタイム定量 |
| 反応タイプ | 1ステップRT-PCR | 1ステップRT-PCR | マルチプレックス1ステップRT-PCR |
| リアルタイムまたはエンドポイント | リアルタイム | リアルタイム | リアルタイム |
| サンプル/ターゲットタイプ | RNA | RNA | RNA |
| シングルプレックスまたはマルチプレックス | シングル | シングル | シングル |
| SYBR Green Iまたは配列特異的プローブ | SYBR Green I | 配列特異的プローブ | 配列特異的プローブ |
| サーマルサイクラー | すべてのリアルタイムサイクラー(LightCycler、Rotor-Gene、ABIなど) | すべてのリアルタイムサイクラー(LightCycler、Rotor-Gene、ABIなど) | マルチプレックスPCR専用のリアルタイムサイクラー(ほとんどのApplied Biosystemsリアルタイムサイクラー、Roche LightCycler 480、Bio-Rad iCycler iQなど) |
| ROXあり/なし | ROXあり | ROXあり | ROXあり/なし |
裏付けデータと数値
1ステップRT-PCRと2ステップ RT-PCRでは同等の結果が得られます。