✓ オンライン注文による24時間年中無休の自動処理システム
✓ 知識豊富で専門的な製品&テクニカルサポート
✓ 迅速で信頼性の高い(再)注文
REPLI-g FFPE Kit (25)
Cat. No. / ID: 150243
✓ オンライン注文による24時間年中無休の自動処理システム
✓ 知識豊富で専門的な製品&テクニカルサポート
✓ 迅速で信頼性の高い(再)注文
特徴
- パラフィン包埋切片から直接全ゲノム増幅
- FFPE組織から精製したDNAの全ゲノム増幅
- DNA収量の標準化と調節が可能:組織切片あたり最高40 µg
- 迅速で簡単なプロトコールで迅速な結果
製品詳細
ゲノム解析のためのゲノムDNA量が不十分であるという問題は、サンプル中の全DNA増幅(whole genome amplification)により解決できます。REPLI-g FFPE KitにはDNA Polymerase、斬新なバッファー、ホルマリン固定パラフィン包埋(FFPE)組織からDNA精製なしに効率的な全ゲノム増幅を実現する試薬が含まれています。組織切片を溶解後、DNA を前処理し、断片化DNAをライゲートします。高性能のREPLI-g テクノロジーを用いて増幅することで長鎖DNAを産生します。
パフォーマンス
増幅時間により2通りのプロトコールがあります。標準的な品質のテンプレートを用いた場合の50 µl の反応あたりの一般的な収量は、標準反応(増幅時間2時間)では10 µg まで、高収量反応(増幅時間8時間)では40 µgまで回収できます。
原理
ゲノム解析において、解析のためのゲノムDNAを十分量確保できない場面によく遭遇します。全ゲノム増幅(WGA)はサンプル内の全DNAを全体的に増幅することにより、このような制限を克服し、同一のDNA検体について全ての解析を行なうのに十分な量のDNAを供給します。
ホルマリン固定パラフィン包埋 (FFPE) 組織サンプルのジェノタイピングは、組織の形態学的変化と特定のゲノム変異を直接結びつけることができます。しかし、ホルマリン固定はDNAに非可逆的な損傷を与え、DNAが断片化するばかりでなくサンプル中の他の生体分子とのクロスリンクが起こります。さらにFFPE組織サンプルからは限られた量のDNAしか抽出できず、増幅なしでは限られた解析しか行なえません。
REPLI-g FFPE Kitは、断片化したDNAを前処理しライゲートする斬新なDNA 処理反応等の操作により、このような問題を克服します(図 " REPLI-g FFPEの原理")。ランダムにライゲートしたDNAの全ゲノム増幅は、高性能のREPLI-gテクノロジーを用いて行なわれます。REPLI-g製品はmultiple displacement amplification(MDA)テクノロジーとユニークな増幅用DNAポリメラーゼを組み合わせたキットです。REPLI-g DNA Polymerase は高い伸長性と相補鎖置換能により、ミスマッチ配列を持つ増幅産物を最小限に抑えて全ゲノム領域に渡り遺伝子座を良好に保持します。そのため、PCRベースのWGA法と比べてより信頼できる結果を実現します。この斬新なDNA処理反応により、断片化の著しいFFPE組織由来DNAにMDAテクノロジーの利点を適用できるようになりました。貴重な試料物質を遺伝子座を良好に保持しつつ増幅することができ、ダウンストリーム解析を制限なく実施できます。
図参照
操作手順
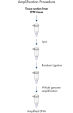
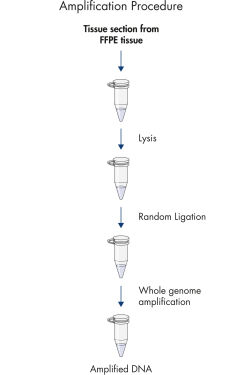
REPLI-g FFPEプロトコールでは、 FFPE組織サンプルからの精製DNAの増幅ができるだけでなく、FFPE組織サンプルから事前にDNA精製せずに直接DNAの増幅が行なえます。組織切片を溶解後、斬新なバッファーおよび酵素を用いてDNAを前処理し、断片化したDNAをライゲートします(フローチャート " REPLI-g FFPE 操作手順"および図 " 高分子のライゲーション産物")。ライゲーション反応により産生した長鎖DNAを高性能のREPLI-gテクノロジーを用いて全ゲノム増幅します。一度増幅したDNAは、精製なしでほとんどのジェノタイピングアッセイに即使用できます。
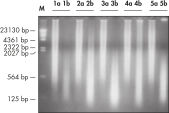
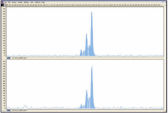
REPLI-g FFPE操作により増幅したDNAは、リアルタイムPCR(例、QuantiFast Kitを使用)、エンドポイントPCR(例、QIAGEN Fast Cycling PCR Kitを使用)に最適であり、増幅するPCRアンプリコンのサイズは開始時のテンプレートの平均フラグメントサイズよりも小さいことが好ましいと考えられます。さらマイクロサテライト解析(図 " 信頼できるマイクロサテライト解析")およびSNPジェノタイピングにも使用できます(REPLI-g FFPEKitで増幅したDNAは、DNAラベリングに 制限酵素処理を必要とするジェノタイピング法には適していません)。
図参照
アプリケーション
REPLI-g FFPE Kitを用いて増幅したDNAは、以下のようなアプリケーションに最適です。
- リアルタイム PCR
- エンドポイントPCR
- SNP ジェノタイピング
- マイクロサテライト解析
裏付けデータと数値
REPLI-g FFPE procedure.
Specifications
| Features | Specifications |
|---|---|
| Yield | 10 µg (2h reaction)-40 µg (8h reaction) |
| Quality assessment | No |
| Starting material | FFPE tissue sections or purified genomic DNA |
| Technology | MDA |
| Maximum input volume | 1 tissue section (10-40 µm thickness) |
| Reaction time | 2-8h (2h: standard; 8h: high-yield reaction) |
| Reaction volume | 50 µl |
| Starting amount of DNA | 100-300 ng* |
| Applications | Genotyping, PCR, Real-time PCR |
| Samples per run (throughput) | medium |
| Amplification | Whole genomic DNA |