Products
特徴
- 細胞に添加し、インキュベートするだけの簡単操作で時間を節約
- より簡単な操作で効率的なトランスフェクション
- 一定した条件下で多数の実験が可能
製品詳細
FlexiTube siRNA PremixはsiRNAとトランスフェクション試薬が最適な割合で配合されています。siRNAと試薬が混和済みなので試薬の混和やコンプレックス形成のための操作が不要で実験時間を短縮できます。さらにFlexiTube siRNA Premixを用いれば、siRNAと試薬の比率を決定するための至適化実験の必要がありません。FlexiTube siRNA Premixはヒト、マウスの遺伝子用があります。
パフォーマンス
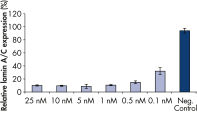
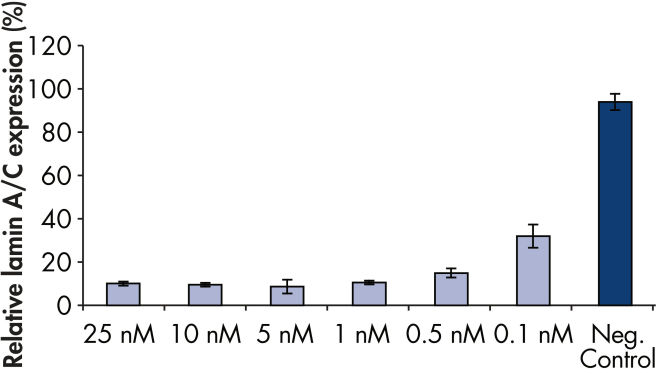
FlexiTube siRNA Premix中のsiRNAと試薬との比率は、高いトランスフェクション効率とノックダウン効率を示します(図 “ 迅速で効率的なノックダウン”および“ ノックダウン後の表現型解析”)。
FlexiTube siRNA Premixに入っているFlexiTube siRNA はQIAGENの高性能なHP OnGurad siRNA Designを用いてデザインされ、高い抑制効果と特異性を実現します。siRNAは膨大なRNAi 実験のデータセットを基にしたニューラルネットワークテクノロジーを用いてデザインされています。その後、適正に評価された重複のない最新の配列データベースと新しい特徴を取り入れたQIAGEN独自の相同性解析ツールを用いてsiRNAデザインとゲノムの他の配列との相同性をチェックします。
性能保証
FlexiTube siRNA Premixesには1回だけ無料で再納品する保証が付いております。同じターゲット遺伝子に対して数種類のFlexiTube siRNA Premixesを注文され、そのうち少なくとも2種類が ≥70%のターゲット遺伝子のノックダウンを実現しなかった場合、QIAGEN は追加料金無しで、2種類のsiRNAを1度だけ再納品します。再納品に際しては適切なトランスフェクション条件下でsiRNAがmRNAレベルでターゲット遺伝子を最低70%ノックダウンできなかったことを証明するデータが必要です。このデータにはトランスフェクション効率、silencingの定量データ、ポジティブコントロールで70%以上のノックダウン効果を示すデータも含まれます。このオファーは製品納入後6か月まで有効です。
図参照
原理
図参照
操作手順
FlexiTube siRNA Premixは細胞へのトランスフェクションへ即使用可能です。細胞に添加しインキュベートするだけの簡単な操作です。通常のトランスフェクション実験はsiRNAを希釈してトランスフェクション試薬と混和、これをインキュベートしてsiRNA試薬のコンプレックスを形成し、このコンプレックスを細胞に添加してインキュベーションを行なう、というステップが必要ですが、これと比較すると、FlexiTube siRNA Premixは時間と労力を大幅に節約できます。
FlexiTube siRNA Premixを用いると、siRNA と試薬の比率を至適化する必要がないのでRNAi実験をすぐに開始できます。FlexiTube siRNA PremixではsiRNA と試薬の比率が最適に混和されているため、多くのトランスフェクションを含む時間のかかる至適化実験は最小限に抑えられるか回避することができます。
1本のFlexiTube siRNA Premixで複数のトランスフェクションが行なえます。これは実験間におけるばらつきを抑え、一定した信頼できる実験結果を実現します。
アプリケーション
FlexiTube siRNA Premixは、少数の標的遺伝子を用いた機能ゲノミクスあるいはパスウェイ解析に最適です。
裏付けデータと数値
Rapid, efficient knockdown.
Specifications
| Features | Specifications |
|---|---|
| Design | HP OnGuard design |
| Target sequence provided | Yes |
| Modification | No |
| siRNA per target gene | variable |
| Format | Tube |
| Number of transfections | 50 standard transfections in 24-well format |
| Guarantee/validation | for validated siRNAs |
| Species | Human, mouse |
| Scale or yield | 0.75 nmol |