QIAprep Spin Miniprep Kit
20 µgまでの分子生物学実験グレードのプラスミドDNA精製用
20 µgまでの分子生物学実験グレードのプラスミドDNA精製用
Cat. No. / ID: 27104
QIAprep Spin Miniprep Kitは高純度なプラスミドDNAあるいはコスミドDNA(最高20 µg)を精製できます。精製DNAはシークエンシングおよびクローニングのようなルーチンの分子生物学アプリケーションに使用できます。High-Yield Supplementary Protocol により、さらに高い収量(最高30 µgまで)を得ることができます。
QIAprep Spin Miniprep Kitでは、分子生物学実験グレードのプラスミドDNAまたはコスミドDNA を最大20 µg 精製することができ、PCR、シークエンシング、クローニングのようなルーチンの分子生物学アプリケーションでの使用に適しています。汎用性のあるQIAprep 2.0 Spin Columns は、マイクロ遠心機または吸引マニホールドでき、QIAcube上で自動化も可能です(図 "QIAprep 2.0 Spin Columns操作オプション A、 B、および C")。吸引法を使用することで、取り扱いが簡便になり、サンプル処理時間が短くなります。QIAprep 2.0 Spin Columns は、QIAvac 24 Plusをはじめとする市販のルアーコネクター付きマニホールドで吸引処理することができます。QIAprep Spin Miniprep Kit はQIAcube 上で自動化も可能です。
| フォーマット: | スピンカラム |
| 精製モジュール: | QIAprep 2.0 Spin Columns |
| 処理数: | 1~24サンプル |
| 操作時間: | 30分で24ミニプレップ |
| 必要な装置: | マイクロ遠心分離機あるいは吸引装置;QIAcubeを用いて完全自動化 |
| ライセート清澄化: | 遠心操作 |
| カラム容量 | 800 µl |
| 最小溶出バッファー量 | 50 µl |
| 高コピープラスミド用培養液量 | 1~500 ml |
| 低コピープラスミド/コスミド用培養液量 | 1~10 ml |
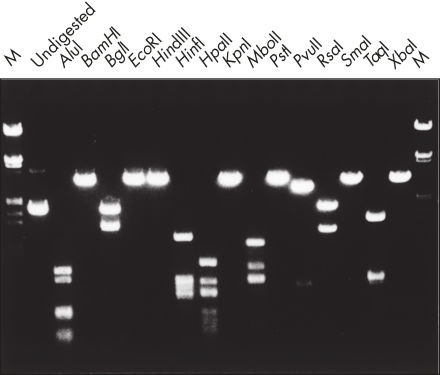
精製したDNAは、制限酵素分解で使用することができます(図 " 様々な制限酵素を用いた解析")。
QIAprep Kit を用いたプラスミド精製法は、結合、洗浄そして溶出という非常にシンプルなものです(フローチャート" QIAprep操作手順" 参照)。まずバクテリアを溶解後、そのライセートを遠心操作により清澄化します。清澄化されたライセートをQIAprep 2.0モジュールにアプライし、プラスミドDNA をシリカメンブレンに結合します。夾雑物を洗い流し、高純度DNAだけを少量の溶出バッファーまたは水で溶出します。
QIAprep Kit は大腸菌からだけではなくビール酵母菌、枯草菌、Agrobacterium tumefaciensからのプラスミドDNA 精製にも利用できます。これらのプロトコールに関しては弊社テクニカルサポートにお問い合わせください。
QIAprep Miniprep Kit は、下記の用途を含むほとんどのアプリケーションに最適な高純度DNAを再現性良く調製します。
| Features | Specifications |
|---|---|
| Applications | Fluorescent and radioactive sequencing (including capillary sequencing), ligation, cloning, transformation, etc. |
| Processing | Manual (vacuum or centrifugation) or automated (QIAcube Connect) |
| Plasmid type | High-copy, low-copy, cosmid DNA |
| Culture volume/starting material | 1–10 mL culture volume |
| Elution volume | 50 µl (minimal) |
| Technology | Silica technology |
| Time per run or prep per run | <30 minutes |
| Yield | <20 µg |
| Samples per run (throughput) | 1–24 samples per run |
| Number of preps per run | 1–24 samples per run |