QIAsymphony DSP Virus/Pathogen Kit
ウイルス核酸あるいはバクテリアDNA の自動精製
ウイルス核酸あるいはバクテリアDNA の自動精製
QIAsymphony DSP Virus/Pathogen Kits とQIAsymphony SP の組み合わせは、多様な検体からウイルス核酸およびバクテリア/病原体由来DNA の自動精製を実現します。キットは200 μl あるいは最大1,000 μl までの検体量に応じて、それぞれMini、Midi のフォーマットがあります。至適化済みのプロトコールにより高純度DNA が高収量で得られ、溶出容量が調節可能なのでダウンストリームアプリケーションごとにDNA 濃度の至適化が簡単にできます。斬新で充填済みの試薬カートリッジによりセットアップ時間を短縮し、マニュアルでの取り扱いは最小限に抑えられます。バーコードによる読み取りにより、試薬のトラッキングを完全に行なえます。
QIAsymphony DSP Virus/Pathogen Kitsは、血清、血漿、CSFからウイルス核酸を精製するために使用するだけではなく、尿や泌尿生殖器のスワブ(子宮頸管や尿道)同様、スワブ、吸引液、気管支肺胞洗浄(BAL)のような呼吸器系検体からウイルス核酸およびバクテリアDNAを精製できます。本キットは、様々なDNAおよびRNAウイルスからの核酸精製またはグラム陰性菌やグラム陽性菌からのバクテリアDNAの精製に用いることができます。
QIAsymphony DSP Virus/Pathogen Kitsは、シリカゲルベースによる効率的で迅速なウイルス/バクテリア核酸精製と磁性粒子による簡便操作を組み合わせています(図“ QIAsymphony SP原理の模式図”)。革新的で即使用可能な試薬カートリッジは、精製工程に必要なすべての試薬が充填済みです。 QIAsymphony SPはカートリッジを自動的に開封し、実験環境からのコンタミのリスクを回避します(図" 充填済みのプレパック試薬カートリッジ”)。至適化済みのプロトコールにより、タンパク質、ヌクレアーゼ、その他の不純物を効率的に除去し、高品質核酸を精製します。QIAsymphony DSPVirus/Pathogen Kitsで精製した核酸はダウンストリームのアプリケーション(PCRなど)ですぐに使用できます。ユーザーは適切な溶出量を選択できます。
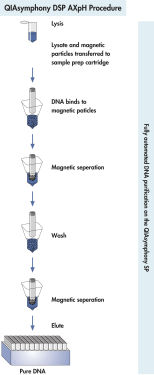
精製操作は感染性の高いサンプルを安全かつ再現性よく取り扱えるようにデザインされ、溶解、結合、洗浄、溶出の4ステップで構成されています(図“ QIAsymphony DSP Virus/Pathogen操作”)。ワークテーブルのセットアップが簡単かつ迅速に行なえ、貴重な時間を節約できます。QIAsymphony SP のボックスに最高2種類の試薬カートリッジを入れるだけで準備完了です。同じキットの試薬カートリッジではなく、別々のキットの試薬カートリッジをセットすれば、一回のランで96サンプルから2種類の精製工程が可能です。選択したサンプル数だけの試薬量が使用されるため、コストを完璧にコントロールできます。
| プロトコール | キット | サンプル | サンプル容量 |
|---|---|---|---|
| Cellfree 200 | QIAsymphony DSP Virus/Pathogen Mini Kit | 血清、血漿、CSF | 200 µll |
| Cellfree 500 | QIAsymphony DSP Virus/Pathogen Midi Kit | 血清、血漿、CSF | 500 μl |
| Cellfree 1000 | QIAsymphony DSP Virus/Pathogen Midi Kit | 血清、血漿、CSF | 1000 μl |
| Complex 200 | QIAsymphony DSP Virus/Pathogen Mini Kit | 呼吸器系検体(BAL、乾燥スワブ、輸送保存液、吸引液、痰)および泌尿生殖器検体(尿、輸送保存液) | 200 μl |
| Complex 400 | QIAsymphony DSP Virus/Pathogen Midi Kit | 呼吸器系検体(BAL、乾燥スワブ、輸送保存液、吸引液、痰)および泌尿生殖器検体(尿、輸送保存液) | 400 μl |
| Complex 800 | QIAsymphony DSP Virus/Pathogen Midi Kit | 呼吸器系検体(BAL、乾燥スワブ、輸送保存液、吸引液、痰)および泌尿生殖器検体(尿、輸送保存液) | 800 μl |
QIAsymphony DSP Virus/Pathogen Kit は様々なDNA およびRNA ウイルス、また病原性細菌を高感度で検出します。直線性のある収量が得られ、ウイルスあるいはバクテリアは力価にかかわらず正確な定量解析が可能です。高品質な核酸は、リアルタイム定量 PCRあるいはRT-PCRを用いた高感度検出を含むすべてのダウンストリームアプリケーションで即使用可能です。