QIAwave Plasmid Miniprep Kit (50)
Cat. No. / ID: 27204
Features
- Plasmid DNA quality and performance identical to the QIAprep Spin Miniprep Kit
- Up to 22% less plastic and up to 14% less cardboard compared to the QIAprep Spin Miniprep Kit
- Reusable Waste Tubes made from 100% post-consumer recycled plastic
- Buffer concentrates that use up to 93% less plastic than our standard buffers
Product Details
The QIAwave Plasmid Miniprep Kit is an eco-friendlier version of our standard QIAprep Spin Miniprep Kit. The QIAwave Kit uses up to 22% less plastic and up to 14% less cardboard than our standard kit and offers waste tubes made from 100% post-consumer recycled plastic that you can reuse throughout the procedure. QIAwave buffers come as concentrates, reducing the amount of plastic by up to 93% per bottle. To save paper, there are no printed protocols in the kit. You can download the protocols from the resources list or scan the QR code inside the kit box. Although the kit packaging and components of our QIAwave Kit may look different, it’s as easy to use as our standard kit, and the chemistry and performance are identical.
Please be aware that you will need sterile glass bottles to store the reconstituted buffers.
In partnership with My Green Lab, we've also assessed the environmental impact of this kit. My Green Lab ACT labels are designed to evaluate and score products on several sustainability criteria. These include:
• Manufacturing
• Responsible chemical management
• Sustainable content within products and packaging materials
• Disposal of the packaging at the end of life
Products are scored from 1 to 10 except for energy and water consumption, which are scored as 1 point per kWh or gallon, respectively. A low score means a lower environmental impact – see figures "QIAwave Plasmid Miniprep Kit ACT environmental impact factor label US 50/ 250, EU 50/ 250 and UK 50/ 250 ").
The QIAwave Plasmid Miniprep Kit is designed to isolate up to 20 μg high-purity plasmid or cosmid DNA for routine molecular biology applications, including fluorescent and radioactive sequencing and cloning. You can achieve even higher yields (up to 30 μg) using the QIAprep High-Yield Supplementary Protocol. We recommend using this kit with the QIAvac 24 Plus for optimal results.
See figures
 QIAwave Plasmid Miniprep Kit (250) ACT environmental impact factor label US.
QIAwave Plasmid Miniprep Kit (250) ACT environmental impact factor label US. QIAwave Plasmid Miniprep Kit (50) ACT environmental impact factor label EU.
QIAwave Plasmid Miniprep Kit (50) ACT environmental impact factor label EU. QIAwave Plasmid Miniprep Kit (250) ACT environmental impact factor label EU.
QIAwave Plasmid Miniprep Kit (250) ACT environmental impact factor label EU. QIAwave Plasmid Miniprep Kit (250) ACT environmental impact factor label UK.
QIAwave Plasmid Miniprep Kit (250) ACT environmental impact factor label UK.
Performance
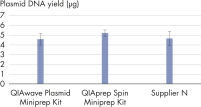
The performance between our QIAwave Plasmid Miniprep Kit and QIAprep Spin Miniprep Kit is identical because the chemistry is the same. We’ve also shown that both kits outperform competitors’ kits (see figure " QIAwave Kit performance").
The QIAwave Plasmid Miniprep Kit allows you to purify up to 20 μg molecular biology grade plasmid DNA or cosmid DNA to use in routine molecular biology applications such as PCR, sequencing and cloning.
The QIAprep 2.0 Spin Columns are so versatile that you can use them in microcentrifuges, on vacuum manifolds or on the QIAcube Connect (see figures "QIAprep 2.0 Spin Column handling options microcentrifuge, vacuum manifolds, and automated system"). The vacuum procedure makes handling simpler and sample processing faster. QIAprep 2.0 Spin Columns can also be vacuum processed using the QIAvac 24 Plus or any other commercial manifold with luer connectors.
| Format | Spin columns |
| Purification module | QIAprep 2.0 Spin Columns |
| Throughput | 1–24 samples |
| Preparation time | 24 minipreps in 30 minutes |
| Equipment required | Microcentrifuge or vacuum manifold; fully automatable using the QIAcube Connect |
| Lysate clearing | Centrifugation |
| Capacity of column reservoir | 800 µL |
| Minimum elution buffer volume | 50 µL |
| Culture volume for high-copy plasmids | 1–5 mL |
| Culture volume for low-copy plasmids/cosmids | 1–10 mL |
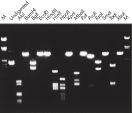
Purified DNA can be used in restriction digestion (see figure " Complete digestion with various restriction enzymes").
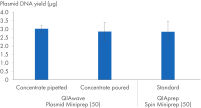
We have also compared plasmid DNA yields obtained using the QIAwave Plasmid Miniprep Kit (50) buffer, prepared by pouring or pipetting, and the QIAprep Spin Miniprep Kit (50) standard buffers. Both methods result in comparable yields as shown in the figure “ Handling of buffer concentrates”.
See figures
Principle
The QIAprep 2.0 Spin Columns contain a unique silica membrane that binds up to 20 μg DNA in the presence of a high concentration of chaotropic salt and allows elution in a small volume of low-salt buffer. QIAprep membrane technology eliminates time-consuming phenol-chloroform extraction and alcohol precipitation, as well as the problems and inconvenience associated with loose resins and slurries. High-purity plasmid DNA eluted from QIAprep 2.0 Spin Columns is immediately ready to use – there is no need to precipitate, concentrate, or desalt.
Procedure
DNA plasmid purification using the QIAwave Plasmid Miniprep follows a simple bind-wash-elute procedure (see flowchart " QIAwave plasmid Miniprep procedure").
1. Lyse bacterial cultures and clear the lysates using centrifugation.
2. Add the cleared lysates to the QIAprep 2.0 Spin Columns. At this point, the plasmid DNA adsorbs to the silica membrane and impurities are washed away.
3. Pure DNA is then eluted in a small volume of elution buffer or water.
In addition to plasmid DNA purification from E. coli, the QIAwave Plasmid Mini Kit can be used to purify plasmid DNA from Saccharomyces cerevisiae, Bacillus subtilis, and Agrobacterium tumefaciens. Contact our Technical Services Team or your local distributor if you need protocols for these applications.
QIAwave buffers come as concentrates that you can easily reconstitute by adding water and/or ethanol; please check the handbook for details. QIAwave QIAprep 2.0 Spin Columns and Waste Tubes come in individual bags and need to be preassembled before you start the protocol. This takes a little extra time, but it does reduce plastic waste.
The QIAwave Plasmid Miniprep Kit can be automated on QIAcube Connect using the QIAprep Spin Miniprep Kit protocols.
See figures
Applications
The QIAwave Plasmid Miniprep Kit provides reproducible yields of high-purity DNA suitable for use in most applications, including:
- PCR
- Restriction digestion
- Ligation and transformation
- Sequencing
- Screening
Supporting data and figures
QIAwave Plasmid Miniprep Kit (50) ACT environmental impact factor label US.
This ACT environmental impact factor label evaluates and scores the QIAwave Plasmid Miniprep Kit (50) for plasmid DNA isolation on several sustainability criteria. Scores are 1–10 except for energy and water consumption, which is scored as 1 point per kWh. or gallon, respectively. A low score means a lower environmental impact. The QIAwave Plasmid Miniprep Kit (50) has a 36.8% lower EIF in the US than the equivalent standard kit, the QIAprep Spin Miniprep Kit (50).

Specifications
| Features | Specifications |
|---|---|
| Applications | Fluorescent and radioactive sequencing (including capillary sequencing), ligation, cloning, transformation etc. |
| Processing | Manual (centrifugation or vacuum) |
| Plasmid type | High-copy, low-copy, cosmid DNA |
| Culture volume/starting material | 1–10 mL culture volume |
| Elution volume | 50 µL (minimal) |
| Technology | Silica technology |
| Time per run or prep per run | <30 minutes |
| Yield | <20 ug |
| Samples per run (throughput) | 1–24 samples per run |
| Number of preps per run | 1–24 samples per run |