✓ 24/7 automatic processing of online orders
✓ Knowledgeable and professional Product & Technical Support
✓ Fast and reliable (re)-ordering
QIAcuity Mycoplasma Quant Kit
Cat. No. / ID: 250261
✓ 24/7 automatic processing of online orders
✓ Knowledgeable and professional Product & Technical Support
✓ Fast and reliable (re)-ordering
Features
- Probe-based assay to quantify and detect 127 different mycoplasma species
- Pharmacopeia-compliant workflow, including 10 different Mycoplasma CFU Standards for validation and for optional positive sensitivity controls.
- Third-party validation report available
Product Details
The QIAcuity Mycoplasma Quant Kit reliably detects and quantifies the presence of mycoplasma rRNA in various sample types. Combined with a recommended sample prep method, we offer a NAT (Nucleic Acid Technique) workflow for mycoplasma testing that is compliant with the European, US and Japanese Pharmacopeia. The robust process eliminates time-consuming cultivation of mycoplasma. The workflow is particularly suited to sample types of varying purity (for example, cell bank samples, in-process samples, such as virus harvest and final lots/batches).
The QIAcuity Mycoplasma Quant Kit provides a consistent, accurate, precise and highly reproducible detection of mycoplasma presence. The detection via rRNA enables a higher sensitivity than using DNA because of the multiple copies present in a single bacterial cell. The assay is still able to detect DNA if the RT-step is skipped, enabling a high degree of flexibility.
The kit is compatible with the QIAcuity Digital PCR System and the QIAcuity Nanoplates.
Learn more about the product from our dPCR specialists. Sign in here and we'll get in touch with you shortly.
Performance
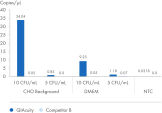
Use the QIAcuity Mycoplasma Quant Kit to detect mycoplasma contamination in cell cultures and pharmaceutical products during the different manufacturing steps. Benefit from a workflow that is fully compliant with the European, US and Japanese Pharmacopeia. Download a full validation report to assess the performance of the kit and its standards. Please note, you must verify the validation for your specific sample matrix before first use.
Principle
The QIAcuity Mycoplasma Quant Kit, together with the QIAcuity Digital PCR System, form a unique solution for detecting and quantifying mycoplasma contamination. The digital PCR-based workflow offers a winning combination of performance, ease of use and compliance with the European Pharmacopeia (EP; chapter 2.6.7), the US Pharmacopeia (UP; chapter <63>), and the Japanese Pharmacopeia (JP; chapter G3-14-170). Besides the 10 mycoplasma species mentioned in the Pharmacopeias, you can detect another 127 mycoplasma species using the QIAcuity Mycoplasma Quant Kit. To assess the performance of the workflow, download a validation report from an independent party, Minerva Biolabs GmbH (Berlin, Germany).
The validated workflow consists of only two steps. First, extract the nucleic acids from the sample, then analyze the nucleic acids via RT-dPCR. With the QIAcuity OneStep Advanced Probe chemistry, you can perform the reverse transcription and PCR in a single step. Although we recommend detecting mycoplasma using an RT-dPCR protocol, you can also directly detect DNA in a digital PCR reaction without the RT-step.
The principle of the dPCR reaction in the nanoplates is described here.
Procedure
The validated workflow consists of two steps in which the nucleic acids are extracted from the sample and subsequently analyzed via RT-dPCR. Using the QIAcuity OneStep Advanced Probe chemistry, the required reverse transcription and PCR are done within a single hands-on step. Although we recommend detecting mycoplasma using the RT-dPCR protocol described below, the assay also detects DNA and can be used in a dPCR reaction without the RT-step.
Sample preparation
To reliably detect mycoplasma contamination, RT- and PCR inhibitors and other impurities should be absent in your sample. As an overall control for inhibitors, you can choose to add the QIAcuity Mycoplasma Internal Control to the lysis buffer before the sample extraction. You can directly use the eluate for mycoplasma detection using the QIAcuity Mycoplasma Quant Kit with the corresponding QIAcuity OneStep Advanced Probe chemistry.
QIAcuity OneStep Advanced Probe Kit
Merge reverse transcription and PCR for detecting mycoplasma contamination without setting up a separate RT-reaction by combining HotStart Reverse Transcription (RT) Enyzme, HotStart QuantiNova DNA Polymerase, proprietary chemical components and the QIAcuity Mycoplasma Assay.
QIAcuity Mycoplasma Assay
The QIAcuity Mycoplasma Assay includes a FAM-labeled 20x primer-probe mix for the detection of a specific mycoplasma rRNA target and a HEX-labeled primer-probe mix for the optional detection of the QIAcuity Mycoplasma Internal Control. We’ve thoroughly tested this duplex reaction for cross-talk. With the assay, you can rely on a single control for RNA extraction, reverse transcription, amplification and detection, eliminating the need for extra reactions.
Applications
QIAcuity Mycoplasma Quant Kit and Standards are designed for detecting and quantifying mycoplasma contamination in cell cultures and biopharmaceutical development processes.
Supporting data and figures
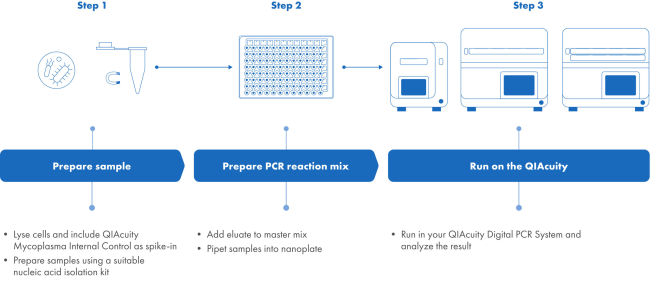
Mycoplasma testing following the QIAcuity Mycoplasma Quant Kit workflow
The Pharmacopeia-compliant NAT (nucleic acid technologies) workflow using the QIAcuity Mycoplasma Quant Kit consists of three steps. Step 1: The sample is processed using an appropriate nucleic acid isolation technique. During the lysis step, the Mycoplasma Internal Control is spiked-in as process control. Step 2: QIAcuity Mycoplasma Quant Master Mix is prepared and dispensed into a preplate. Eluates are added and the reaction mix the preplate is transferred to a 26k Nanoplate. Step 3: The nanoplate is placed into the QIAcuity Digital PCR instrument and the RT-PCR run is started. The results are analyzed using the QIAcuity Software Suite.