RNeasy Mini Kit (50)
Cat. No. / ID: 74104
Features
- Fast procedure delivering high-quality total RNA in minutes
- Ready-to-use RNA for high performance in any downstream application
- Consistent RNA yields from small to large amounts of starting material
- No phenol/chloroform extraction, no CsCl gradients, no LiCl or ethanol precipitation
- High-throughput processing in 96-well format and automatable protocols
Product Details
RNeasy Kits are the gold standard for total RNA isolation. They provide fast purification of high-quality RNA from small to large amounts of cells, tissues, and yeast using silica membrane RNeasy spin columns or 96-well plates. Tissue samples can be conveniently stabilized using RNAprotect Tissue Reagent or Allprotect Tissue Reagent, and efficiently disrupted using a TissueRuptor II or TissueLyser II or LT system. The RNeasy 96 Kit enables high-throughput purification of total RNA from up to 96 cultured cell samples using silica membrane RNeasy 96 plates. A dedicated RNeasy QIAcube Kit enables automated purification of 1–12 samples on the QIAcube Connect.
Performance
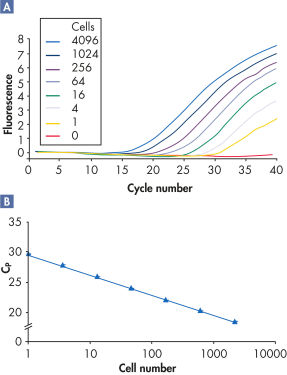
RNeasy Kits and QIAwave RNA Kits deliver highly reproducible yields of total RNA from small to large samples. Total RNA can reliably be purified from small numbers of cells, including a single cell, as well as from small amounts of standard tissues (see figures "Reliable RNA isolation from a single cell", "Highly reproducible yields for sensitive applications" and "High-quality total RNA from fine needle aspirates") using the RNeasy Micro Kit. RNA purified using the RNeasy Micro Kit provides maximum sensitivity with precious samples in quantitative gene expression analyses, such as real-time RT-PCR, by efficient on-column digestion of genomic DNA (see figure "Efficient on-column removal of genomic DNA").
Total RNA is easily purified from animal or human cells, animal or human tissues, and yeast (see table “Total RNA yields obtained with RNeasy Kits” and figure "RT-PCR of RNA from as few as 100 cells").
The QIAwave RNA Mini Kit gives you highly reproducible yields of total RNA from animal or human cells, animal or human tissues, and yeast (see table “Total RNA yields obtained with RNeasy and QIAwave RNA Kits"). The performance between our QIAwave RNA Mini Kit and RNeasy Mini Kit is identical because the chemistry is the same. We’ve also shown that both kits outperform competitors’ kits (see figure "QIAwave RNA Mini Kit performance").
Total RNA yields obtained with RNeasy and QIAwave RNA Kits
| Source | Starting material | Average yield | ||||||||
| Animal cells | Micro | Mini/QIAwave Mini | Midi | Maxi | 96-well | Micro | Mini/QIAwave Mini | Midi | Maxi | 96-well |
| LMH | 5 x 105 | 1 x 106 | 7 x 107 | 5 x 108 | 1 x 105 | 6 µg | 12 µg | 850 µg | 5.7 mg | 1.3 µg* |
| HeLa | 5 x 105 | 1 x 106 | 7 x 107 | 4 x 108 | 1 x 105 | 5 µg | 15 µg | 1000 µg | 6.0 mg | 1.6 µg* |
| COS-7 | 5 x 105 | 1 x 106 | 3 x 107 | 1.8 x 108 | 1 x 105 | 17.5 µg | 35 µg | 950 µg | 5.8 mg | 3.1 µg* |
| Lymphocytes (unstimulated) |
5 x 105 | 1 x 106 | 1 x 108 | 5 x 108 | – | – | 0.5 µg | 50 µg | 0.3 mg | – |
| Huh7 | 5 x 105 | – | – | – | 1 x 105 | 7.5 µg | – | – | – | 2.0 µg* |
| Jurkat | 5 x 105 | – | – | – | 1 x 105 | – | – | – | – | 1.4 µg* |
| Mouse tissue | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Liver | 5 mg | 10 mg | 200 mg | 1 g | – | 15 µg | 40 µg | 700 µg | 3.6 mg | – |
| Lung | 5 mg | 10 mg | 100 mg | 0.5 g | – | 5 µg | 10 µg | 130 µg | 0.6 mg | – |
| Spleen | 5 mg | 10 mg | 200 mg | 1 g | – | 15 µg | 35 µg | 600 µg | 3.2 mg | – |
| Yeast cells | ||||||||||
| S. cerevisiae | – | 1 x 107 | 2 x 108 | 1 x 109 | – | – | 25 µg | 450 µg | 2.4 mg | – |
The dedicated RNeasy Mini QIAcube Kit, including rotor adapters preloaded with RNeasy spin columns and elution tubes, delivers greater convenience and time savings (see figure "Significant time savings with dedicated QIAcube Kits").
Total RNA purified with the RNeasy Maxi Kit is of high quality and is suitable for many downstream applications (see figure "High-quality RNA from a variety of samples"). Total RNA is easily purified with the RNeasy Maxi Kit from large amounts of starting material including animal or human cells, animal or human tissues, and yeast cells (see table “Total RNA yields obtained with RNeasy Kits”).
The RNeasy 96 system provides fast and reproducible total RNA purification for high-throughput gene expression profiling. The RNA is suitable for sensitive applications such as quantitative, real-time RT-PCR and microarray analysis (see figure "High-quality RNA for sensitive analysis of a low-copy transcript"). Sample sizes range from 10 to 5 x 105 cells (see figure "RT-PCR of RNA from as few as 100 cells"), and high-quality RNA can be purified from large numbers of samples (see figure "Reproducible yields of high-quality RNA"). Individual variations are low throughout the entire purification process; TaqMan® threshold-cycle values are easily achieved at the end of the process with a coefficient of variation (CV) less than 3% using the QuantiTect Probe RT-PCR Kit (see figure "Repeatability of fully automated RNA purification").
See figures
 Highly reproducible yields for sensitive applications.
Highly reproducible yields for sensitive applications.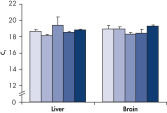 High-quality total RNA from fine needle aspirates.
High-quality total RNA from fine needle aspirates.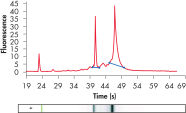 Efficient on-column removal of genomic DNA.
Efficient on-column removal of genomic DNA. RT-PCR of RNA from as few as 100 cells.
RT-PCR of RNA from as few as 100 cells.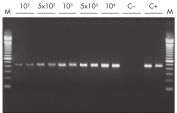 QIAwave Kit performance.
QIAwave Kit performance.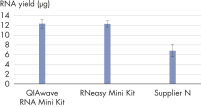 Significant time savings with QIAcube Kits.
Significant time savings with QIAcube Kits.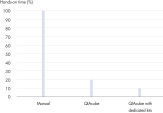 High-quality RNA from a variety of samples.
High-quality RNA from a variety of samples.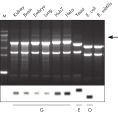 High-quality RNA for sensitive analysis of a low-copy transcript.
High-quality RNA for sensitive analysis of a low-copy transcript. Reproducible yields of high-quality RNA.
Reproducible yields of high-quality RNA. Repeatability of automated RNA purification.
Repeatability of automated RNA purification.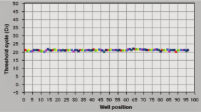
Principle
RNeasy Kits and QIAwave RNA Kits allow efficient purification of total RNA from small to large amounts of starting material, including samples such as microdissected tissues and fine needle aspirates, from milligram amounts of fibrous tissues including heart and muscle tissue, and from small numbers of cells down to single cells (e.g., FACS sorted cells). For microdissected FFPE tissues, we recommend the RNeasy FFPE Kit. RNeasy technology simplifies total RNA isolation from cells, tissues and yeast by combining the stringency of guanidine-isothiocyanate lysis with the speed and purity of silica-membrane purification. RNeasy and QIAwave RNA Kits provide the highest-quality RNA with minimum copurification of DNA.
The QIAwave RNA Mini Kit is an eco-friendlier version of our standard RNeasy Mini Kit. Reducing plastic and cardboard usage by up to 60% and 57%, respectively, this version incorporates waste tubes made from 100% post-consumer recycled plastic that can be reused during the procedure. The QIAwave buffers are provided as concentrates, decreasing plastic use by up to 90% per bottle. Despite the visual differences, the QIAwave Kit remains user-friendly, with chemistry and performance identical to the standard kit's.
In partnership with My Green Lab, we've also assessed the environmental impact of the RNeasy Mini Kit and the QIAwave RNA Mini Kit. My Green Lab ACT (accountability, consistency, and transparency) environmental impact factor labels are designed to evaluate and score products on several sustainability criteria. These include:
- Manufacturing
- Responsible chemical management
- Sustainable content within products and packaging materials
- Disposal of the packaging at the end of life
Products are scored from 1 to 10 except for energy and water consumption, which are scored as 1 point per kWh or gallon, respectively. A low score means a lower environmental impact (see figures "Mini Kit ACT environmental impact factor label US ( 50/ 250), EU ( 50/ 250) and UK ( 50/ 250)".
See figures
 RNA Mini Kits (250) ACT environmental impact factor label US.
RNA Mini Kits (250) ACT environmental impact factor label US. RNA Mini Kit (50) ACT environmental impact factor label EU.
RNA Mini Kit (50) ACT environmental impact factor label EU. RNA Mini Kits (250) ACT environmental impact factor label EU.
RNA Mini Kits (250) ACT environmental impact factor label EU. RNA Mini Kits (50) ACT environmental impact factor label UK.
RNA Mini Kits (50) ACT environmental impact factor label UK. RNA Mini Kits (250) ACT environmental impact factor label UK.
RNA Mini Kits (250) ACT environmental impact factor label UK.
Procedure
RNeasy technology simplifies total RNA isolation (see table “Amount of starting material for RNeasy procedures”). Samples are first lysed and then homogenized. Ethanol is added to the lysate to provide ideal binding conditions. The lysate is then loaded onto the RNeasy silica membrane and RNA binds to the silica membrane, and all contaminants are efficiently washed away. For certain RNA applications that are sensitive to very small amounts of DNA, the residual amounts of DNA remaining can be removed using a convenient on-column DNase treatment. Pure, concentrated RNA is eluted in water. A variety of special application protocols is also available.
Automated processing on the QIAcube Connect
The QIAcube Connect uses advanced technology to process QIAGEN spin columns, enabling seamless integration of automated, low-throughput sample prep into laboratory workflows. All steps in the purification procedure are fully automated — and up to 12 samples can be processed per run. The QIAcube Connect together with the dedicated RNeasy Mini QIAcube Kit provides fast, easy, and convenient RNA purification.
High-throughput processing in 96-well format
The RNeasy 96 system provides a fast and efficient procedure. Cells can be grown and directly lysed in 96-well cell-culture dishes. After transfer of the lysates to the wells of the RNeasy 96 plate, RNA binds to the silica membrane in each well, and contaminants are washed away. Pure RNA is then eluted in RNase-free water into individual collection tubes suitable for long-term storage and is ready to use for any experiment. The RNeasy 96 Kit includes protocols for total RNA isolation, cytoplasmic RNA isolation, and RNA cleanup. RNeasy 96 plates can be processed on the QIAvac 96 vacuum manifold and/or the 96-Well-Plate Centrifuge System.
| Amount of starting material |
RNeasy Micro Kit | RNeasy Mini Kit/QIAwave RNA Mini Kit | RNeasy Mini QIAcube Kit | RNeasy Midi Kit | RNeasy Maxi Kit | RNeasy 96 Kit |
| Cells | <5 x 105 cells | 10 to 1 x 107 animal or human cells | 1 x 107 cells | 5 x 106 to 1 x 108 animal or human cells | Up to 5 x 108 animal or human cells | 10–5 x 105 cells |
| Tissue | <5 mg tissue | 0.5–30 mg animal or human tissues | 30 mg tissue | 20–250 mg animal or human tissues | 150 mg to 1 g tissue | – |
| Yeast | <5 x 107 yeast cells | – | 2 x 107 to 5 x 108 yeast cells | 2.5 x 109 yeast cells | – |
Applications
RNA purified with RNeasy and QIAwave RNA technology has A260/280 ratios of 1.9–2.1 (measured in 10 mM Tris·Cl, pH 7.5) and is ideal for use in all applications. Downstream applications include:
- RNA-seq
- Quantitative, real-time RT-PCR
- Real-time RT-PCR starting with as little as one cell
- End-point RT-PCR
- Northern, dot, and slot blotting
- Array analysis
- Poly A+ RNA selection
Comparison of RNeasy and QIAwave RNA Kits
| Features | RNeasy Micro Kit | RNeasy Mini Kit/QIAwave RNA Mini Kit | RNeasy Mini QIAcube Kit | RNeasy Midi Kit | RNeasy Maxi Kit | RNeasy 96 Kit |
| Applications | RNA-seq, quantitative real-time RT-PCR, end-point RT-PCR, Northern dot and slot blotting, Microarray analysis |
RNA-seq, quantitative real-time RT-PCR, end-point RT-PCR, Northern dot and slot blotting, Microarray analysis |
RNA-seq, quantitative real-time RT-PCR, end-point RT-PCR, Northern dot and slot blotting, Microarray analysis |
RNA-seq, quantitative real-time RT-PCR, end-point RT-PCR, Northern dot and slot blotting, Microarray analysis |
RNA-seq, quantitative real-time RT-PCR, end-point RT-PCR, Northern dot and slot blotting, Microarray analysis |
RNA-seq, quantitative real-time RT-PCR, end-point RT-PCR, Northern dot and slot blotting, Microarray analysis |
| Elution volume | 10–14 µl | 30–100 µl | 30–100 µl | 300–500 µl | 800–2400 µl | 45–140 µl |
| Format | Spin column | Spin column | Spin column, preloaded into rotor adapters | Spin column | Spin column | 96-well plate |
| Main sample type | Tissue, cells | Tissue, cells, yeast | Tissue, cells, yeast | Tissue, cells, yeast | Tissue, cells, yeast | Cultured cells |
| Processing | Manual (centrifugation) | Manual (centrifugation) or automated (QIAcube Connect) | Automated (QIAcube Connect) | Manual (centrifugation) | Manual (centrifugation) | Manual (centrifugation or vacuum) |
|
RNA type |
Total RNA (> 200 nt) | Total RNA (> 200 nt) | Total RNA (> 200 nt) | Total RNA (> 200 nt) | Total RNA (> 200 nt) | Total RNA (> 200 nt) |
| Sample amount | <5 mg tissue, <5 x 105 cells |
0.5–30 mg tissue, 10 to 1 x 107 animal or human cells |
0.5–30 mg tissue, 1 x 107 cells |
20–250 mg tissue, 5 x 106 to 1 x 108 animal or human cells |
150 mg – 1 g tissue, Up to 5 x 108 animal or human cells |
5 x 105 |
| Technology | Silica technology | Silica technology | Silica technology | Silica technology | Silica technology | Silica technology |
| Time per run or per prep | 30–40 minutes | 20 minutes | – | <1 hour | 1 hour | 30–40 minutes |
| Yield | Varies | Varies | Varies | Varies | Varies | 1.3–3.1 µg |
Supporting data and figures
Reliable RNA isolation from a single cell.