Products
Caractéristiques
- Procédure de travail complète conforme au marquage CE-IVD (QIAsymphony RGQ) (1-4)
- Fiabilité élevée grâce au contrôle interne et aux contrôles positifs (1-4)
- Détection très sensible dès 43,2 copies/ml (kit QS-RGQ) (3)
- Même profil de température pour de multiples virus (kits RG et QS-RGQ uniquement)
Détails produit
Les kits artus HSV-1/2 PCR sont des kits de détection moléculaire prêts à l’emploi destinés à la PCR en temps réel. Les kits comprennent tous les réactifs nécessaires, optimisés pour la détection rapide et sensible de l’ADN spécifique du HSV-1/2 isolé dans le LCR (kits RG), le LCR et le plasma (kits QS-RGQ), ou le sérum, le plasma, le LCR et les écouvillons (kits LC et TM) dans le cadre d’un diagnostic in vitro. Les kits artus HSV-1/2 LC, RG et TM PCR utilisent des techniques manuelles ou automatiques de préparation des échantillons et des techniques manuelles de préparation des tests. Le kit artus HSV-1/2 RG PCR est également disponible en tant que kit EASYartus HSV-1/2 RG PCR combiné au kit EZ1 DSP Virus pour la purification automatisée de l’acide nucléique viral. Le kit artus HSV-1/2 QS-RGQ est intégré au système QIAsymphony RGQ, système complet automatisé, de l’échantillon à la détection de pathogènes, et ne doit pas être utilisé dans le cadre d’une préparation manuelle de tests.
Performances
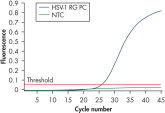
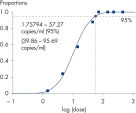
Afin de garantir une sensibilité maximale, les kits artus HSV-1/2 ont été optimisés de manière à détecter l’ADN du HSV-1/2 en faible quantité. La sensibilité analytique du kit artus HSV-1/2 QS-RGQ est de 57,3 copies/ml pour le HSV-1 et de 65,7 copies/ml pour le HSV-2 compte tenu de la purification à partir du LCR et de la préparation de la PCR réalisées sur le système QIAsymphony RGQ (voir figure « Détection très sensible de l’ADN du HSV-1 »).
| Kit | artus HSV-1/2 RG PCR | artus HSV-1/2 QS-RGQ |
|---|---|---|
| Types d’échantillons validés | Liquide céphalo-rachidien (LCR) humain | Liquide céphalo-rachidien humain (LCR) ou plasma EDTA |
| Sensibilité analytique | 0,12 copies/μl pour le HSV-1 0,16 copies/μl pour le HSV-2 en PCR | 57,3 copies/ml pour le HSV-1 (LCR) 65,7 copies/ml pour le HSV-2 (LCR) 57,5 copies/ml pour le HSV-1 (plasma) 43,2 copies/ml pour le HSV-2 (plasma) compte tenu de la purification |
| Spécificité | Testé avec le HSV-1 (souches HF, KOS, MacIntyre) et le HSV-2 (souches HG-52, G et MS) | Testé avec le HSV-1 (souches HF, KOS, MacIntyre) et le HSV-2 (souches HG-52, G et MS) |
Principe
Les kits artus HSV-1/2 PCR se fondent sur l’amplification et la détection simultanée d’une région spécifique du génome du HSV-1 et du HSV-2 à partir d’une PCR en temps réel. Ils permettent des niveaux élevés de spécificité, de sensibilité (voir figure « Détection très sensible de l’ADN de HSV-1 ») et de reproductibilité.
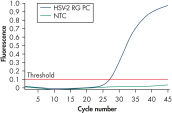
Les kits incluent des contrôles positifs pour le HSV-1 et le HSV-2 (voir figures « Détection du contrôle positif HSV-1 » et « Détection du contrôle positif HSV-2 »). Par ailleurs, les kits incluent un second système d’amplification hétérologue destiné à l’identification de toute inhibition possible de la PCR. Cette détection s’effectue par le biais d’un contrôle interne dans un canal de fluorescence distinct de celui de la PCR analytique.
| Kit | artus HSV-1/2 RG PCR | artus HSV-1/2 QS-RGQ |
|---|---|---|
| Types d’échantillons validés | Liquide céphalo-rachidien (LCR) humain | Liquide céphalo-rachidien humain (LCR) ou plasma EDTA |
| Amplicon | Région de 154 paires de bases du gène UL5 | Région de 154 paires de bases du gène UL5 |
Procédure
Les kits artus HSV-1/2 PCR incluent tous les réactifs nécessaires, optimisés pour une détection et une différenciation fiables de l’ADN du HSV-1/2. Il suffit d’ajouter de l’ADN matrice au master mix PCR prêt à l’emploi et à la solution Mg et de lancer la réaction sur le thermocycleur en temps réel approprié à l’aide du programme de cycle optimisé décrit dans le manuel du kit.
Profils de températures courants pour de multiples tests en une seule étape
Les profils de températures des kits artus HSV-1/2 LC PCR, RG PCR et QS-RGQ correspondent aux profils des kits artus LC PCR, RG PCR et QS-RGQ pour le CMV, l’EBV, le VZV et (kits RG et QS-RGQ uniquement) le BK virus. Par conséquent, ces tests de PCR peuvent être réalisés et analysés en une seule étape.
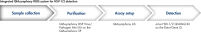
Système complet automatisé, de l’échantillon à la détection du HSV-1/2
La chaîne complète QIAsymphony RGQ pour la détection du HSV-1/2 se compose du module QIAsymphony SP pour la préparation des échantillons, du module QIAsymphony AS pour la préparation des réactions d’amplification, et du kit artus HSV-1/2 QS-RGQ pour la détection sur l’instrument Rotor-Gene Q. Ce système intégré permet la détection fiable des agents pathogènes par l’intermédiaire d’une procédure de travail complète certifiée CE-IVD (voir figure « Système intégré QIAsymphony RGQ pour la détection du HSV-1/2 »).
Recommandations pour la purification d’ADN viral dans le cadre de l’utilisation des kits artus LC, RG et TM PCR
Pour la purification de l’ADN, il est recommandé d'utiliser uniquement le kit EZ1 DSP Virus pour la purification automatisée des acides nucléiques viraux. Le kit artus HSV-1/2 RG PCR est également disponible en tant que kit EASYartus HSV-1/2 RG PCR certifié CE-IVD, notamment en tant que kit EZ1 DSP Virus pour la purification automatisée des acides nucléiques viraux.
Applications
Les kits artus HSV-1/2 PCR permettent une détection rapide et sensible de l’ADN du HSV-1/2 dans le plasma EDTA. Les kits assurent la discrimination du HSV-1 et du HSV-2 au moyen de sondes spécifiques au génotype.
Le kit artus HSV-1/2 QS-RGQ est conçu pour une utilisation avec le système QIAsymphony RGQ et permet une procédure de travail complète conforme au marquage CE-IVD de l’échantillon à la détection de l’ADN du HSV et à la discrimination du HSV - 1 et du HSV-2.
Données et illustrations utiles
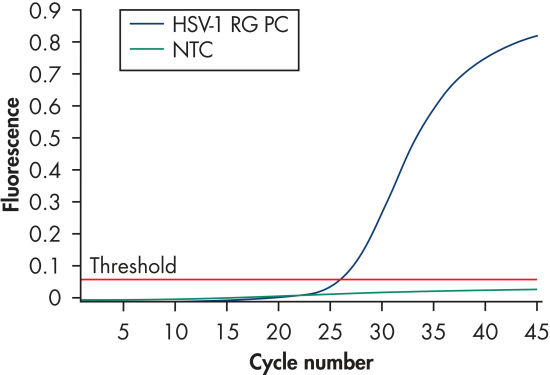
Detection of the HSV-1 positive control.
Specifications
| Features | Specifications |
|---|---|
| Quantitative/qualitative | Quantitative/qualitative |
| Sample type | Serum, plasma, cerebrospinal fluid (CSF), swabs |
| RUO/CE/ASR | CE |
| Recommended sample prep | QIAamp DNA Mini Kit, QIAamp UltraSens Virus Kit, EZ1 DSP Virus Kit |
| Thermal cycler | ABI PRISM 7000, 7900HT SDS, LightCycler, Rotor-Gene Q Instruments |
| What detected | Herpes Simplex Virus 1 and 2 DNA |