QIAstat-Dx Analytical Module
Cat. No. / ID: 9002814
Features
- Uses real-time PCR to deliver comprehensive results for up to 160 patient samples per day with 8 Analytical Modules
- Easily view Ct values and amplification curves for all detected pathogens
- Single-step cartridge preparation with no precision pipetting required and unique direct swab protocol
- Load the full capacity of cartridges in just a few minutes and safely dispose of all used cartridges from the waste tray in one quick step
Product Details
QIAstat-Dx simplifies infectious disease diagnostics so you can get faster results for your patients. All you need are a patient sample, a QIAstat-Dx assay cartridge and one of our intuitive QIAstat-Dx instruments.
QIAstat-Dx Rise is our higher-throughput powerhouse, built to fit the needs of high-demand institutions. Combined with QIAstat-Dx assay cartridges, QIAstat-Dx Rise uses multiplex PCR technology – also known as syndromic testing – to quickly survey many different pathogens in a single patient sample. The cartridges come ready with all reagents preloaded and use our trusted assay chemistry. The system includes random access and batch testing options, with a daily capacity of 160 samples using 8 Analytical Modules. The QIAstat-Dx Rise also includes a multi-test front drawer that can accommodate up to 18 cartridges at the same time, and a waste drawer to automatically collect used cartridges.
Each instrument is made up of two components: 1) A QIAstat-Dx Rise Base, which contains the intuitive touch-screen interface and robotic system that moves and scans the cartridges within the device and 2) Between 1 through 8 Analytical Modules, letting you test up to eight samples at once.
With QIAstat-Dx Rise in your lab, you can:
- Gain time away from your instruments: Batch process up to 16 cartridges using 8 Analytical modules.
- Process priority tests fast: Get random access through 2 additional urgent testing slots.
- Load and go: Load the full capacity of cartridges in just a few minutes and safely dispose of used tests in one step.
Plus, every QIAstat-Dx Analytical Module is compatible with both QIAstat-Dx Analyzer 2.0 and QIAstat-Dx Rise. Easily update your QIAstat-Dx testing format based on your evolving testing needs. Click here to more about QIAstat-Dx Analyzer 2.0, our modular system perfect for labs of every size.
Principle
QIAstat-Dx Rise, combined with QIAstat-Dx assay cartridges, uses real-time PCR to detect pathogen nucleic acids in human biological samples. The instrument and cartridges are designed as a closed system that contain on board all necessary reagents, enabling hands-off sample preparation. Detected real-time amplification signals are interpreted by the integrated software and are reported via an intuitive user interface.
Procedure
Preparing a QIAstat-Dx cartridge using liquid transfer or dry swab sample application is efficient and fast. And once the cartridges are ready, it takes just a few minutes to load the full capacity of cartridges and start a run on QIAstat-Dx Rise.
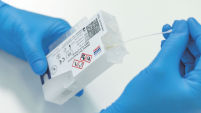
Loading a sample into the QIAstat-Dx cartridge requires less than one minute hands-on time with no precision pipetting.
Using the provided transfer pipette, the user applies a liquid sample to the main port of the QIAstat-Dx cartridge (see Applying a liquid sample to the QIAstat-Dx cartridge). Alternatively for our respiratory assay, a dry swab sample may be directly applied to the cartridge ( Applying a dry swab to the QIAstat-Dx cartridge).
Following the instructions on the QIAstat-Dx Rise touchscreen display, the user loads the prepared cartridges into the instrument’s loading tray (see Adding cartridges to the QIAstat-Dx Rise input tray).
The QIAstat-Dx Rise will automatically scan the sample ID and cartridge barcodes, or the user can manually scan the barcodes. Once the user starts the run, the QIAstat-Dx Rise will autonomously process the samples.
The input tray also offers random and continuous access, meaning an urgent test can be added to an ongoing run at a moment’s notice.
Once the run is finished, the used cartridges are automatically placed in the dedicated waste tray so they can be disposed of safely and quickly.
See figures
Applications
QIAstat-Dx Respiratory SARS-CoV-2 Panel (V2): Qualitative test to analyze 23 viral and bacterial respiratory targets for common pathogens causing respiratory infections, including SARS-CoV-2.
QIAstat-Dx Respiratory SARS-CoV-2 Panel (V2) – 23 bacterial and viral pathogens
| Viruses | Bacteria | |
|---|---|---|
| Influenza A | Parainfluenza virus 1 | |
| Influenza A subtype H1N1/2009 | Parainfluenza virus 2 | |
| Influenza A subtype H1 | Parainfluenza virus 3 | |
| Influenza A subtype H3 | Parainfluenza virus 4 | Mycoplasma pneumoniae |
| Influenza B | Respiratory syncytial virus A/B | Legionella pneumophilia |
| Coronavirus 229E | Human metapneumovirus A/B | Bordetella pertussis |
| Coronavirus HKU1 | Adenovirus | Chlamydophila pneumoniae |
| Coronavirus NL63 | Bocavirus | |
| Coronavirus OC43 | Rhinovirus/Enterovirus* | |
| SARS-CoV-2 |
*Enterovirus and Rhinovirus are both detected, but not differentiated, with the QIAstat-Dx Respiratory SARS-CoV-2 Panel.
The QIAstat-Dx Respiratory SARS-CoV-Panel (V2) is currently available in select countries.
QIAstat-Dx Gastrointestinal Panel 2: Qualitative test to analyze 22 of the most common pathogens causing gastrointestinal infections, including differentiation of Shiga-like toxin producing E. coli (STEC) stx1 and stx2.
QIAstat-Dx Gastrointestinal Panel 2 – 23 targets, corresponding to 22 bacterial, viral and parasitic pathogens
| Bacteria | Viruses | Parasites | |
|---|---|---|---|
| Campylobacter (C. jejuni, C. upsaliensis, C. coli) | Salmonella spp. | Adenovirus F40/41 | Cryptosporidium |
| Clostridium difficile (toxin A/B) | Shiga-like toxin-producing E. coli (STEC) stx1 and stx2 (including specific identification of E. coli O157 serogroup within STEC)** | Astrovirus | Cyclospora cayetanensis |
| Enteroaggregative E. coli (EAEC) | Vibrio vulnificus | Norovirus (GI, GII) | Entamoeba histolytica |
| Shigella/Enteroinvasive E. coli (EIEC) | Vibrio parahaemolyticus | Rotavirus A | Giardia lamblia |
| Enteropathogenic E. coli (EPEC) | Vibrio cholerae | Sapovirus (GI, GII, GIV, GV) | |
| Enterotoxigenic E. coli (ETEC) lt/st | Yersinia enterocolitica | ||
| Plesiomonas shigelloides |
**The stx1 and stx2 toxin genes of STEC are reported separately by the QIAstat-Dx Gastrointestinal Panel 2.
| Features | Specifications |
|---|---|
| Configuration | Modular syndromic testing system with QIAstat-Dx Rise Base, up to 8 QIAstat-Dx Analytical Modules and 1 QIAstat-Dx Rise Mobile Table (optional). |
| Instrument dimensions (WxHxD) | QIAstat-Dx Rise Base (door closed): 580 x 1280 x 810 mm (22.8 x 50.4 x 31.9 in) QIAstat-Dx Rise Base (door open): 1500 mm x 1280 x 810 mm (59 x 50.4 x 31.9 in) For installation and service interventions, 1.5 m of space around the instrument is required. |
| Weight | QIAstat-Dx Rise Base: 130 kg QIAstat-Dx Analytical Module: 16 kg QIAstat-Dx Rise Mobile Table (load-bearing capacity): 300 kg |
| Power requirements | 200–240 VAC |
| Recommended clearance | Left side: 80 cm Top: 32 cm Front: 140 cm |
| Operating conditions | Specifications |
| Air temperature | 15–28 ̊C |
| Relative humidity | 20–80% |
| Altitude | 0–2200 m |
| Place of operation | Laboratory (inside) |
Supporting data and figures
Adding cartridges to the QIAstat-Dx Rise input tray
Following the instructions on the QIAstat-Dx Rise touchscreen display, the user loads the prepared cartridges into the instrument’s loading tray. The QIAstat-Dx Rise will automatically scan the sample ID and cartridge barcodes. Once the user starts the run, the QIAstat-Dx Rise will autonomously process the samples.