QIAcuity One, 2plex Device
Cat. No. / ID: 911001
Características
- Sistema completamente integrado
- Formato escalable (instrumentos de 1, 4 y 8 placas)
- Capacidades avanzadas de multiplexado (hasta 5-plex)
- Rendimiento flexible de la muestra
- Resultados completos en unas 2 horas
Detalles del producto
El QIAcuity Digital PCR System está diseñado para ofrecer resultados de cuantificación multiplexados y precisos para detección de mutaciones, variación en el número de copias (CNV), estudios de expresión génica, análisis de modificación de genes y mucho más. Este sistema basado en nanoplacas integra a la perfección un flujo de trabajo de dPCR estándar de división en partes, termociclado y obtención de imágenes en una plataforma automatizada que no requiere supervisión con un tiempo de manipulación mínimo.
El sistema se utiliza junto con las nanoplacas, los reactivos y los ensayos QIAcuity.
Explore la demostración virtual para obtener más información sobre QIAcuity.
Rendimiento
El QIAcuity Digital PCR System permite que la cuantificación absoluta sea accesible y asequible para todos los laboratorios. La automatización sin necesidad de supervisión integra y agiliza todo el flujo de trabajo de PCR digital de división en partes, termociclado y obtención de imágenes en un único instrumento con un tiempo de manipulación mínimo. Adaptar sus ensayos de qPCR actuales al QIAcuity Digital PCR System es también muy fácil. No es necesario realizar cambios en la manipulación de las placas que provienen de qPCR, lo que garantiza una configuración rápida del ensayo y la obtención de resultados en unas 2 horas.
Características y especificaciones de los instrumentos QIAcuity
| Característica | QIAcuity One | QIAcuity Four | QIAcuity Eight |
|---|---|---|---|
| Placas procesadas | 1 | 4 | 8 |
| Canales de detección (multiplexado) | 2 o 5 | 5 | 5 |
| Termocicladores | 1 | 1 | 2 |
| Tiempo necesario para obtener el resultado | Unas 2 h | Primera placa en unas 2 h Cada ∼80 min la placa siguiente | Primera placa en unas 2 h Cada ∼40 min la placa siguiente |
| Rendimiento (muestras procesadas en una jornada de trabajo) | Hasta 384 (96 pocillos) Hasta 96 (24 pocillos) | Hasta 672 (96 pocillos) Hasta 168 (24 pocillos) | Hasta 1248 (96 pocillos) Hasta 312 (24 pocillos) |
Principio
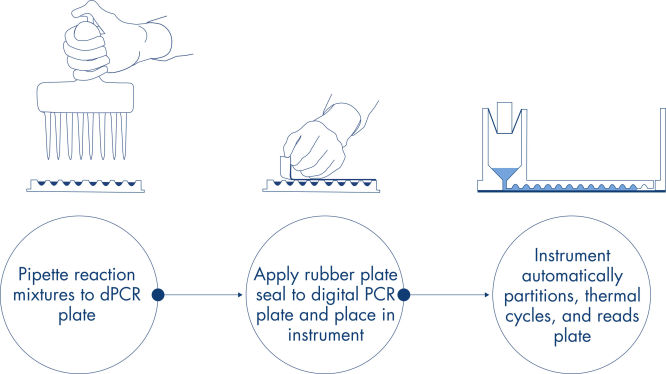
En solo 3 simples pasos, puede obtener el resultado de dPCR que desea en unas 2 horas: pipetee y cargue, realice el experimento y analice los resultados.
El principio de la reacción de dPCR en las nanoplacas se describe aquí.
Procedimiento
Al igual que en los experimentos de qPCR, la preparación de las muestras incluye la transferencia de mezcla maestra, sondas y cebadores a una nanoplaca de 96 o 24 pocillos, seguida de la adición de las muestras. El sistema integra funciones de división, termociclado y obtención de imágenes en un solo instrumento completamente automatizado que permite a los usuarios obtener los resultados de las muestras en menos de 2 horas. Es posible realizar un análisis en el paquete de software, que proporciona la concentración en copias por microlitro de la secuencia de blancos y también para el control de calidad como muestras positivas o NTC. Este análisis también se puede extender a ordenadores remotos dentro de la misma red de área local (LAN).
Aplicaciones
Los instrumentos QIAcuity, junto con las QIAcuity Nanoplates y los QIAcuity PCR kits, posibilitan las aplicaciones de PCR digital, entre las que se incluyen:
- Detección de mutaciones poco frecuentes
- Análisis de variación en el número de copias
- Análisis de expresión génica
- Detección de microrganismos patógenos
- Genotipado
- Investigación sobre miARN
- Terapia celular y génica
Software
El QIAcuity Software Suite que se incluye con el instrumento y se instala en un ordenador independiente permite controlar uno o varios instrumentos QIAcuity, ya sea que estén conectados directamente a un instrumento o que utilicen una red de área local existente (LAN). Al utilizar el QIAcuity Software Suite, es posible definir los experimentos de PCR digital, las muestras y las mezclas de reacciones, asignarlas a Nanoplates y transferirlas al instrumento QIAcuity. Tras la serie, es posible analizar los datos, crear informes y exportar los datos para el análisis externo. El software proporciona varias funcionalidades de molde que permiten acceder fácilmente a las distribuciones de placas o parámetros de la serie de placas que se repiten, transformando aún más su experiencia de PCR digital.
Si está integrado en una red de área local, el ordenador aloja la función del QIAcuity Software Suite como un servidor al que se puede acceder fácilmente a través de una red LAN a otros ordenadores que funcionan como clientes. De esta manera, varios usuarios pueden acceder al software desde otras salas u oficinas y analizar los datos a través de un navegador estándar sin tener que instalar el software en varios ordenadores o acceder a los datos e intercambiarlos a través de conexiones a Internet.
Servicios
Proteja su instrumento con una gran variedad de soluciones de servicio de QIAGEN. Obtenga más información sobre un acuerdo de servicio específico que se adapte a sus necesidades.
Datos y cifras de respaldo
Flujo de trabajo simple y rápido basado en la placa
Planes de servicio
QIAcuity One 2plex Full Agreement
Cat. No. / ID: 9245362
QIAcuity One Preventive Subscription
Cat. No. / ID: 9245355
QIAcuity Four Preventive Subscription
Cat. No. / ID: 9245356
QIAcuity Eight Preventive Subscription
Cat. No. / ID: 9245357
QIAcuity One 2plex Core Agreement
Cat. No. / ID: 9245358
QIAcuity One 5plex Core Agreement
Cat. No. / ID: 9245359
QIAcuity Four Core Agreement
Cat. No. / ID: 9245360
QIAcuity Eight Core Agreement
Cat. No. / ID: 9245361
QIAcuity One 5plex Full Agreement
Cat. No. / ID: 9245363
QIAcuity Four Full Agreement
Cat. No. / ID: 9245364
QIAcuity Eight Full Agreement
Cat. No. / ID: 9245365
QIAcuity One Installation & Training
Cat. No. / ID: 9245352
QIAcuity Four Installation & Training
Cat. No. / ID: 9245353
QIAcuity Eight Installation & Training
Cat. No. / ID: 9245354