QuantiTect Probe RT-PCR Kit (200)
Cat. No. / ID: 204443
Características
- Detección de dianas con pocas copias muy sensible
- qRT-PCR mediante sondas específicas de secuencia o SYBR Green
- Cuantificación exacta sobre varios logaritmos del molde
- Sin necesidad de optimizar la reacción y las condiciones de termociclado
- Detección del gen de referencia y de hasta 3 dianas en el mismo tubo
Detalles del producto
Los QuantiTect RT-PCR Kits permiten la cuantificación sensible de las dianas de ARN mediante la real-time PCR de un paso utilizando sondas específicas de secuencia o detección SYBR Green I. Los kits también permiten la cuantificación fiable de hasta 5 dianas de ARN en un único tubo mediante la RT-PCR de un paso en tiempo real y múltiple. La combinación de un hot start y un sistema de tampón de PCR exclusivo en la mezcla maestra lista para usar garantiza una qRT-PCR de alta sensibilidad en cualquier termociclador en tiempo real sin necesidad de optimización. La mezcla dNTP incluye dUTP, lo que permite el tratamiento opcional con UNG. Por motivos de conveniencia, las mezclas maestras en los kits de RT-PCR de un paso de QuantiTect pueden almacenarse a una temperatura de 2-8 °C.
Hay dos formatos de kits disponibles para la RT-PCR múltiple utilizando sondas específicas de secuencia: el QuantiTect Multiplex RT-PCR Kit para termocicladores que necesitan un colorante ROX para la normalización de la fluorescencia y el QuantiTect Multiplex RT-PCR NoROX Kit para el resto de termocicladores. El QuantiTect SYBR Green RT-PCR Kit se suministra con una mezcla de RT optimizada para una transcripción inversa sensible y eficaz en una gran variedad de cantidades de moldes de ARN.
Rendimiento
La HotStarTaq DNA Polymerase incluida en los kits de RT-PCR de un paso de QuantiTect aumenta la especificidad de la reacción de PCR al proporcionar el hot start más estricto en comparación con otras polimerasas.
El QuantiTect SYBR Green RT-PCR Kit contiene una mezcla única de transcriptasas inversas que proporciona una síntesis de ADNc sensible y eficaz en una gran variedad de cantidades de moldes de ARN y permite una cuantificación específica en un amplio intervalo lineal (consulte la figura “ Resultados comparables en la RT-PCR de un paso y de dos pasos”).
El QuantiTect Probe RT-PCR Kit contiene un tampón de RT-PCR exclusivo que favorece la hibridación altamente específica de sondas y cebadores al molde de PCR. La HotStarTaq DNA Polymerase y la composición exclusiva del tampón de RT-PCR permiten que el QuantiTect Probe RT-PCR Kit proporcione una cuantificación sensible de dianas de ARN de pocas copias, así como una cuantificación exacta en un amplio intervalo lineal (consulte la figura “ Alta sensibilidad y eficacia y amplio rango dinámico”).
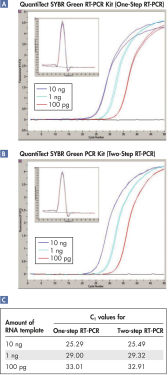
Llevar a cabo la transcripción inversa y la PCR secuencialmente en el mismo tubo no merma la sensibilidad, tal y como demuestran los valores de CT comparables a aquellos obtenidos en la RT-PCR en tiempo real de dos pasos (consulte las figuras “ RT-PCR de un paso con rendimiento comparable a la RT-PCR de dos pasos: A” y “ RT-PCR de un paso con rendimiento comparable a la RT-PCR de dos pasos: B”, y la tabla “RT-PCR de un paso con rendimiento comparable a la RT-PCR de dos pasos”).
RT-PCR de un paso con rendimiento comparable a la RT-PCR de dos pasos.
| Cantidad de ADNc/ARN |
Valor de CT medio (RT-PCR de dos pasos) |
Valor de CT medio (RT-PCR de un paso) |
|---|---|---|
| 100 ng | 24,66 | 24,66 |
| 10 ng | 28,15 | 27,84 |
| 1 ng | 31,42 | 31,49 |
| 0,1 ng | 34,80 | 34,56 |
| 0,01 ng | 37,78 | 37,66 |
La detección sensible de tan solo 10 copias de la secuencia diana (consulte la figura “ Detección de hasta 10 copias del ARN diana en PCR doble”) puede lograrse en la qRT-PCR múltiple con los QuantiTect Multiplex RT-PCR Kits. La cuantificación relativa precisa de la expresión de un gen se consigue a través de la cuantificación de la expresión tanto del gen diana como de un gen de control endógeno en el mismo pocillo o tubo. Con los QuantiTect Multiplex RT-PCR Kits, los valores de CT de las dianas en una reacción múltiple son equivalentes a aquellos obtenidos en los experimentos de control, donde las dianas se amplifican en reacciones separadas (consulte la figura “ Amplificación comparable en la PCR triple y las PCR simples”). Esto demuestra que las dianas diferentes en la misma reacción múltiple se amplifican de forma eficaz y sensible sin influirse entre ellas.
Ver figuras
 Alta sensibilidad y eficacia y amplio rango dinámico.
Alta sensibilidad y eficacia y amplio rango dinámico. RT-PCR de un paso con rendimiento comparable a la RT-PCR de dos pasos: A.
RT-PCR de un paso con rendimiento comparable a la RT-PCR de dos pasos: A. RT-PCR de un paso con rendimiento comparable a la RT-PCR de dos pasos: B.
RT-PCR de un paso con rendimiento comparable a la RT-PCR de dos pasos: B. Detección de hasta 10 copias del ARN diana en PCR doble.
Detección de hasta 10 copias del ARN diana en PCR doble. Amplificación comparable en la PCR triple y las PCR simples.
Amplificación comparable en la PCR triple y las PCR simples.
Principio
Los QuantiTect RT-PCR Kits contienen una mezcla maestra optimizada y lista para usar para una cuantificación en tiempo real muy específica y sensible de dianas de ARN utilizando SYBR Green I o sondas específicas de secuencia. El colorante fluorescente SYBR Green I en la mezcla maestra de QuantiTect SYBR Green RT-PCR permite el análisis de muchas dianas diferentes sin necesidad de sintetizar sondas marcadas como específicas de la diana (consulte la tabla “Componentes del 2x QuantiTect SYBR Green RT-PCR Kit”).
Componentes del 2x QuantiTect SYBR Green RT-PCR Kit
| Componente | Características | Beneficios |
|---|---|---|
| HotStarTaq DNA Polymerase | Activación de 15 min a 95 ºC | Preparación de reacciones de qPCR a temperatura ambiente |
| QuantiTect SYBR Green RT-PCR Buffer | Combinación equilibrada de iones de NH4+ y K+ | La hibridación de cebadores específicos garantiza la fiabilidad de los resultados de la PCR |
| Mezcla dNTP | Incluye dUTP, que sustituye parcialmente al dTTP y permite el tratamiento opcional de las reacciones con UNG | Elimina la contaminación causada por el arrastre de productos de PCR mediante el tratamiento opcional con UNG |
| Colorante SYBR Green I | Produce una señal fluorescente intensa al unirse al ADN bicatenario | Cuantificación muy sensible |
| Colorante ROX | Para la normalización de las señales fluorescentes en instrumentos de Applied Biosystems y, opcionalmente, de Agilent | Cuantificación precisa en termocicladores que requieren colorante ROX. No interfiere en las reacciones en otros termocicladores en tiempo real |
| Omniscript y Sensiscript Reverse Transcriptases (transcriptasas inversas Omniscript y Sensiscript) | Mezcla especial de enzimas con alta afinidad por el ARN | El ARN puede transcribirse, incluso a través de estructuras secundarias complejas |
Los QuantiTect Probe RT-PCR Kits contienen una mezcla maestra optimizada y lista para usar para la cuantificación en tiempo real muy específica y sensible de dianas de ARN utilizando sondas específicas de secuencia (consulte la tabla “Componentes del 2x QuantiTect Probe RT-PCR Kit”). Los kits están diseñados para usarse con todos los tipos de sondas específicas de secuencia, incluidas las sondas de hidrólisis (p. ej., TaqMan® y otras sondas con doble marcado), sondas FRET y balizas moleculares.
Componentes del 2x QuantiTect Probe RT-PCR Kit
| Componente | Características | Beneficios |
|---|---|---|
| HotStarTaq DNA Polymerase | Activación de 15 min a 95 ºC | Preparación de reacciones de qPCR a temperatura ambiente |
| QuantiTect Probe RT-PCR Buffer | Combinación equilibrada de iones de NH4+ y K+ | La hibridación de cebadores específicos garantiza la fiabilidad de los resultados de la PCR |
| Mezcla dNTP | Incluye dUTP, que sustituye parcialmente al dTTP y permite el tratamiento opcional de las reacciones con UNG | Elimina la contaminación causada por el arrastre de productos de PCR mediante el tratamiento opcional con UNG |
| Colorante SYBR Green I | Produce una señal fluorescente intensa al unirse al ADN bicatenario | Cuantificación muy sensible |
| Colorante ROX | Para la normalización de las señales fluorescentes en instrumentos de Applied Biosystems y, opcionalmente, de Agilent | Cuantificación precisa en termocicladores que requieren colorante ROX. No interfiere en las reacciones en otros termocicladores en tiempo real |
| Omniscript y Sensiscript Reverse Transcriptases (transcriptasas inversas Omniscript y Sensiscript) | Mezcla especial de enzimas con alta afinidad por el ARN | El ARN puede transcribirse, incluso a través de estructuras secundarias complejas |
La cuantificación relativa precisa de la expresión de un gen se consigue a través de la cuantificación de la expresión tanto del gen diana como de un gen de control endógeno en el mismo pocillo o tubo. La mezcla maestra optimizada en el QuantiTect Multiplex RT-PCR Kit garantiza que los productos de PCR de una reacción múltiple se amplifiquen con la misma eficacia y sensibilidad que los productos de PCR en la reacción de amplificación simple correspondiente (consulte la tabla “Componentes del 2x QuantiTect Multiplex RT-PCR Kit”). Con el kit se pueden detectar tan solo 10 copias de una diana.
Componentes del 2x QuantiTect Multiplex RT-PCR Kit
| Componente | Características | Beneficios |
|---|---|---|
| HotStarTaq DNA Polymerase | Activación de 15 min a 95 ºC | Preparación de reacciones de qPCR a temperatura ambiente |
| QuantiTect Multiplex RT-PCR Buffer | Combinación equilibrada de iones de NH4+ y K+ | La hibridación de cebadores específicos garantiza la fiabilidad de los resultados de la PCR |
| Factor MP sintético | Análisis múltiple fiable de hasta 4 genes en el mismo tubo | |
| Mezcla dNTP | Incluye dUTP, que sustituye parcialmente al dTTP y permite el tratamiento opcional de las reacciones con UNG | Elimina la contaminación causada por el arrastre de productos de PCR mediante el tratamiento opcional con UNG |
| Colorante ROX* | Para la normalización de las señales fluorescentes en instrumentos de Applied Biosystems y, opcionalmente, de Agilent | Cuantificación precisa en termocicladores que requieren colorante ROX. No interfiere en las reacciones en otros termocicladores en tiempo real |
| Omniscript y Sensiscript Reverse Transcriptases (transcriptasas inversas Omniscript y Sensiscript) | Mezcla especial de enzimas con alta afinidad por el ARN | El ARN puede transcribirse, incluso a través de estructuras secundarias complejas |
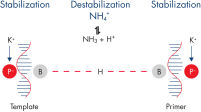
La combinación equilibrada de iones de K+ y NH4+ en el tampón de PCR de los QuantiTect RT-PCR Kits –junto con el Factor MP sintético del tampón de RT-PCR QuantiTect Multiplex– favorece la hibridación de cebadores específicos y permite un alto nivel de especificidad y sensibilidad en la RT-PCR (consulte la figura “ Hibridación de cebadores específicos”). Además, una mezcla optimizada de transcriptasas inversas permite la síntesis de ADNc de una gran variedad de cantidades de moldes de ARN, mientras que la HotStarTaq DNA Polymerase proporciona un riguroso hot start, lo que impide la formación de productos no específicos.
Las mezclas maestras del QuantiTect RT-PCR también contienen dUTP, lo que permite el pretratamiento con uracilo N-glicosilasa (UNG) antes de iniciar la PCR, que garantiza que las reacciones de PCR posteriores no se vean afectadas por productos de PCR contaminantes.
Ver figuras
Procedimiento
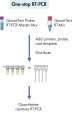
Los QuantiTect RT-PCR Kits no hacen necesaria la optimización de las condiciones de reacción, lo que puede ser tedioso y demandar mucho tiempo. Simplemente, añada los cebadores y el molde a la mezcla maestra lista para usar del QuantiTect SYBR Green RT-PCR –o los cebadores, la sonda y el molde a la mezcla maestra lista para usar del QuantiTect Probe RT-PCR– e inicie la reacción (consulte el organigrama “ RT-PCR de un paso con SYBR Green” y “ RT-PCR de un paso con sondas específicas de secuencia”). Siga el protocolo del manual de uso para obtener resultados rápidos y fiables en cualquier termociclador en tiempo real. Si es necesario, se puede realizar el pretratamiento de las reacciones con uracilo N-glicosilasa (UNG) para eliminar el arrastre de productos de PCR de otras reacciones.
Se garantizan unos resultados muy específicos en el análisis de expresión génica cuando se utilizan los QuantiTect SYBR Green RT-PCR Kits en combinación con los QuantiTect Primer Assays. Se trata de conjuntos de cebadores de genoma completo validados por vía bioinformática destinados a la detección de transcritos de muestras humanas, de ratón, rata y muchas otras. Los QuantiTect Primer Assays se pueden pedir fácilmente en línea en GeneGlobe.
El manual de uso de los QuantiTect Multiplex RT-PCR Kits contiene un protocolo único que puede utilizarse con todos los termocicladores en tiempo real disponibles e incorpora una lista de los colorantes recomendados. Los kits están disponibles con colorante de referencia pasivo ROX en la mezcla maestra o sin él, lo que permite su uso en prácticamente cualquier termociclador en tiempo real (consulte la tabla “Elegir el QuantiTect Multiplex RT-PCR Kit correcto”). Gracias a las concentraciones optimizadas de ROX, se logra la detección incluso de números de copias bajos a través del análisis automático de los datos.
Elegir el QuantiTect Multiplex RT-PCR Kit correcto
| Colorante ROX | Kit | Termocicladores compatibles |
|---|---|---|
| Se suministra en la mezcla maestra | QuantiTect Multiplex RT-PCR Kit | Termocicladores de Applied Biosystems |
| No se suministra en la mezcla maestra | QuantiTect Multiplex RT-PCR NR Kit | Termocicladores Rotor-Gene y termocicladores de Bio-Rad, Cepheid, Eppendorf, Roche, Agilent y otros proveedores |
Ver figuras
Aplicaciones
Los QuantiTect RT-PCR Kits pueden utilizarse para el análisis de la expresión génica de las dianas de ARN en cualquier termociclador en tiempo real. Esto incluye instrumentos de Applied Biosystems, Bio-Rad, Cepheid, Eppendorf, Roche y Agilent. Para el Rotor-Gene Q y otros termocicladores Rotor-Gene, recomendamos el uso del Rotor-Gene Probe RT-PCR Kit, del Rotor-Gene Multiplex RT-PCR Kit o del Rotor-Gene SYBR Green RT-PCR Kit, que se han desarrollado especialmente para el termociclado rápido en esos instrumentos.
| Características | QuantiTect SYBR Green RT-PCR Kit | QuantiTect Probe RT-PCR Kit | QuantiTect Multiplex RT-PCR Kits |
|---|---|---|---|
| Aplicaciones | Cuantificación de dianas de ARN en tiempo real | Cuantificación de dianas de ARN en tiempo real | Cuantificación de dianas de ARN en tiempo real en un formato múltiple |
| Tipo de reacción | RT-PCR de un paso | RT-PCR de un paso | RT-PCR de un paso múltiple |
| En tiempo real o punto final | En tiempo real | En tiempo real | En tiempo real |
| Tipo de muestra/diana | ARN | ARN | ARN |
| Único o múltiple | Único | Único | Único |
| SYBR Green I o sondas específicas de secuencia | SYBR Green I | Sondas específicas de secuencia | Sondas específicas de secuencia |
| Termociclador | Todos los termocicladores en tiempo real (p. ej., LightCycler, Rotor-Gene, ABI) | Todos los termocicladores en tiempo real (p. ej., LightCycler, Rotor-Gene, ABI) | Termocicladores en tiempo real dedicados a la PCR múltiple (p. ej., la mayoría de termocicladores en tiempo real de Applied Biosystems, Roche LightCycler 480, y Bio-Rad iCycler iQ) |
| Con o sin ROX | Con ROX | Con ROX | Con o sin ROX |
Datos y cifras de respaldo
Resultados comparables en la RT-PCR de un paso y de dos pasos.