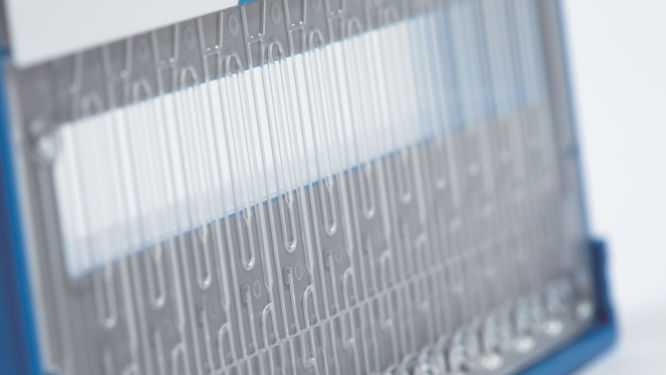
NeuMoDx Cartridge
Cat. No. / ID: 100100
Features
- Flexible assay testing, with true continuous random-access that is scalable to meet your needs
- Room-temperature-stable reagents minimize the need for cold-chain shipment and storage*
- Timely and reliable results available in 50–90 minutes
- Intuitive three-step workflow with minimal hands-on time
- Run IVD and laboratory-developed assays on a single platform using the same reagents and easy, reproducible workflow
* Refer to the product Information for User for exact requirements.
Product Details
Assays are performed on the high-throughput NeuMoDx platforms, which deliver improved performance and increased efficiency by eliminating the waste associated with technologies that required reconstitution of lyophilized reagents. NeuMoDx Molecular Systems simplifies infectious disease diagnostics so you can get faster results to physicians caring for patients.
Principle
The NeuMoDx Molecular System provides automated extraction of nucleic acids from multiple specimen types, as well as automated real-time amplification and detection of target nucleic acid sequences by fluorescence-based, real-time PCR. Our proprietary and unitized microfluidic cartridge features autonomous lanes, allowing simultaneous processing of different assays.
Our unique integration of robotics and advanced microfluidics reduces operation to three simple steps, providing industry-leading usability. These capabilities dramatically improve lab productivity and the ability to provide clinicians with critical information in a timely manner.
A broad IVD menu and room-temperature stable reagents and consumables offer unmatched flexibility while minimizing waste associated with reagent preparation, hands-on time and batch testing. The flexibility of the system allows the consolidation of your infectious disease portfolio onto one system. The versatile NeuMoDx Molecular Systems can also be used as an open system to process lab-developed-tests (LDTs).
The NeuMoDx Cartridge is a proprietary consumable used for the efficacious extraction, purification, amplification and detection of nucleic acids on the NeuMoDx Systems. The NeuMoDx Cartridge is universally used for all tests processed on NeuMoDx Systems for in vitro diagnostic use.
The NeuMoDx Extraction Plate contains a proprietary, dried reagent used for the efficient extraction of nucleic acids on the NeuMoDx Systems in conjunction with other NeuMoDx reagents, such as NeuMoDx lysis buffers, NeuMoDx Wash Reagent and NeuMoDx Release Reagent. The NeuMoDx Extraction Plate is universally used for all tests processed on the NeuMoDx Systems and is formulated to perform both RNA and DNA extraction.
The NeuMoDx Wash Reagent is a proprietary reagent used for the efficacious extraction of nucleic acids on the NeuMoDx Systems in conjunction with other NeuMoDx reagents, such as the NeuMoDx Extraction Plate, NeuMoDx lysis buffers, and NeuMoDx Release Reagent. The NeuMoDx Wash Reagent is universally used for all tests run on the NeuMoDx Systems.
The NeuMoDx Release Reagent is a proprietary reagent used for the efficacious extraction of nucleic acids on the NeuMoDx Systems in conjunction with other NeuMoDx reagents, such as the NeuMoDx Extraction Plate, NeuMoDx lysis buffers and NeuMoDx Wash Reagent.
The NeuMoDx Viral Lysis Buffer is intended for the pretreatment of respiratory specimens suspected to be positive for SARS in UTM-RT or equivalent prior to processing on the NeuMoDx Systems.
The NeuMoDx Vantage Viral Lysis Buffer is intended for the pretreatment of respiratory specimens suspected to be positive for Flu A, Flu B, RSV, and SARS in UVTRT, BD UVT or Biologos Bio-VTM media prior to processing on the NeuMoDx Systems.
The NeuMoDx Viral Lysis Buffer and NeuMoDx Vantage Viral Lysis Buffer are intended for use by trained clinical laboratory personnel specifically instructed and trained in the techniques of real-time PCR and in vitro diagnostic procedures and/or NeuMoDx Molecular Systems. The NeuMoDx Viral Lysis Buffer and NeuMoDx Vantage Viral Lysis Buffer are not intended for self-testing or point-of-care use.
The NeuMoDx Biohazard Waste Bag is an accessory product used to contain the biohazardous waste generated by the NeuMoDx Systems. The NeuMoDx Biohazard Waste Bag is universally used for all tests run on the NeuMoDx Systems.
The NeuMoDx Biohazard Tip Waste Bag is an accessory product used to contain the tips generated by the NeuMoDx 96 Molecular System. The NeuMoDx Biohazard Tip Waste Bag is used only for tests run on the NeuMoDx 96 Molecular System.
NeuMoDx lysis buffers are proprietary buffers used for the efficacious extraction of nucleic acids from unprocessed clinical or biological specimens on the NeuMoDx Systems when used in conjunction with other NeuMoDx reagents, such as NeuMoDx Extraction Plate, NeuMoDx Wash Reagent and the NeuMoDx Release Reagent, which are used for all tests processed on the NeuMoDx Systems. NeuMoDx lysis buffers can be used for extraction of nucleic acids from clinical or biological specimens when used in combination with specified NeuMoDx test strips.
Applications
The NeuMoDx accessories, reagents and consumables are designed to be used with the NeUMoDx Molecular Systems for in vitro diagnostic use.