QIAprep Spin Miniprep Kit – Plasmid Purification
Zur Aufreinigung von bis zu 20 µg molekularbiologischer Plasmid-DNA
Zur Aufreinigung von bis zu 20 µg molekularbiologischer Plasmid-DNA
Cat. No. / ID: 27104
Das QIAprep Spin Miniprep Kit ist für die Isolierung von hochreiner Plasmid- oder Cosmid-DNA konzipiert und bietet bis zu 20 µg Ausbeute zur Verwendung in molekularbiologischen Anwendungen wie Sequenzierung und Klonierung. Mit dem QIAprep High-Yield Supplementary Protocol können sogar noch höhere Ausbeuten (bis zu 30 µg) erzielt werden. Das Kit ist auf dem QIAcube Connect automatisierbar.
Möchten Sie das QIAprep Spin Miniprep Kit ausprobieren? Fordern Sie ein Angebot für ein Test-Kit an.
Für optimale Ergebnisse empfehlen wir, dieses Kit zusammen mit dem QIAvac 24 Plus System zu verwenden.
Das QIAprep Spin Miniprep Kit oder das QIAwave Plasmid Miniprep Kit ermöglicht die Aufreinigung von bis zu 20 µg Plasmid-DNA oder Cosmid-DNA in Molekularbiologie-Qualität zur Verwendung in molekularbiologischen Routineanwendungen wie PCR, Sequenzierung und Klonierung. Die vielseitigen QIAprep 2.0 Spin Columns können entweder in Mikrozentrifugen, auf Vakuumverteilern oder im QIAcube verwendet werden (siehe Abbildungen „Handhabungsoptionen A, B und C der QIAprep 2.0 Spin Columns“). Das Vakuumverfahren ermöglicht eine einfachere Handhabung und schnellere Probenverarbeitung. QIAprep 2.0 Spin Columns können mit dem QIAvac 24 Plus oder jedem anderen handelsüblichen Verteiler mit Luer-Anschlüssen vakuumverarbeitet werden. Das QIAprep Spin Miniprep Kit kann nun auch vollautomatisch auf dem QIAcube Connect eingesetzt werden (siehe Abbildung „ QIAcube Connect“).
Das QIAwave Plasmid Miniprep Kit und das QIAprep Spin Miniprep Kit bieten aufgrund der ähnlichen Chemie eine identische Leistung. Vergleichsdaten zeigen, dass beide Kits die Wettbewerberprodukte übertreffen (siehe Abbildung „Leistung des QIAwave Kit“).
| Format | Spin-Säulen |
| Aufreinigungsmodul | QIAprep 2.0 Spin Columns |
| Durchsatz | 1–24 Proben |
| Vorbereitungszeit | 24 Minipräps in 30 Minuten |
| Erforderliche Arbeitsmittel | Mikrozentrifuge oder Vakuumverteiler; vollständig automatisierbar mit QIAcube Connect |
| Lysatbereinigung | Zentrifugation |
| Fassungsvermögen des Säulenreservoirs | 800 µl |
| Minimales Elutionspuffervolumen | 50 µl |
| Kulturvolumen für Plasmide mit hoher Kopienzahl | 1–5 ml |
| Kulturvolumen für Plasmide mit geringer Kopienzahl/Cosmide | 1–10 ml |
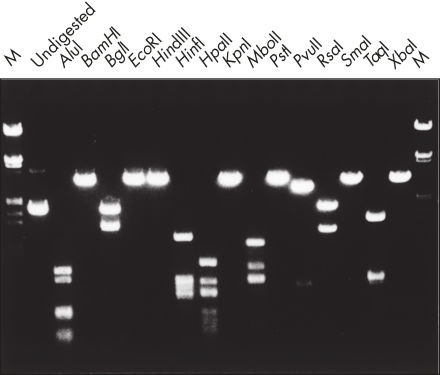
Aufgereinigte DNA kann für den Restriktionsverdau verwendet werden (siehe Abbildung „ Kompletter Verdau mit verschiedenen Restriktionsenzymen“).
QIAprep 2.0 Spin Columns enthalten eine einzigartige Silikamembran, die bei Vorliegen einer hohen Konzentration an chaotropem Salz bis zu 20 μg DNA bindet und die Elution in einem kleinen Volumen an Niedrigsalzpuffer erlaubt. Mit der QIAprep Membrantechnologie entfallen die zeitaufwändige Phenol-Chloroform-Extraktion und Alkoholausfällung sowie die Probleme und Nachteile, die mit losen Harzen und Aufschlämmungen einhergehen. Die aus den QIAprep 2.0 Spin Columns eluierte hochreine Plasmid-DNA ist sofort einsatzbereit und muss nicht ausgefällt, konzentriert oder entsalzt werden.
Zur schnelleren und komfortableren Bearbeitung und Analyse der Proben enthält das Kit einen Gelladefarbstoff. Der GelPilot Ladefarbstoff enthält 3 Markierungsfarbstoffe (Xylencyanol, Bromphenolblau und Orange G), die eine Optimierung der Laufzeit des Agarosegels ermöglichen und verhindern, dass kleinere DNA-Fragmente zu weit migrieren (siehe Abbildung „ GelPilot Ladefarbstoff“).
Als nachhaltigere Alternative bieten wir das QIAwave Plasmid Miniprep Kit an. Bei diesem Kit ist der Plastik- und Kartonverbrauch um bis zu 22 % bzw. 14 % reduziert. Es verfügt über Abfallröhrchen aus 100 % recyceltem Kunststoff, die während des Verfahrens erneut verwendet werden können. Die QIAwave Puffer liegen in konzentrierter Form vor, was den Kunststoffverbrauch pro Flasche um bis zu 93 % reduziert. Trotz des unterschiedlichen Aussehens ist das QIAwave Kit genauso benutzerfreundlich und verfügt über die identische Chemie und Leistung wie das Standardkit.
Wichtig: Für die Lagerung der rekonstituierten Puffer benötigen Sie sterile Glasflaschen.
Gemeinsam mit My Green Lab haben wir die Umweltauswirkungen des QIAprep Spin Miniprep Kit (50/250) und des QIAwave Plasmid Miniprep Kit (50/250) bewertet. My Green Lab ACT-Labels wurden entwickelt, um Produkte nach verschiedenen Nachhaltigkeitskriterien zu beurteilen und zu bewerten. Das sind:
• Herstellung
• Verantwortungsvoller Umgang mit Chemikalien
• Nachhaltige Inhaltsstoffe bei Produkten und Verpackungsmaterialien
• Entsorgung der Verpackung am Ende des Lebenszyklus
Die Produkte werden von 1–10 bewertet, mit Ausnahme des Energie- und Wasserverbrauchs, der mit 1 Punkt/kWh bzw. Gallone (3,785 Liter) bewertet wird. Je niedriger die Punktzahl, desto geringer die Umweltbelastung – (siehe Abbildungen „Plasmid Miniprep Kit ACT Umweltverträglichkeitslabel USA ( 50/ 250), EU ( 50/ 250) und UK ( 50/ 250)).“
 Plasmid Miniprep Kits (50) ACT environmental impact factor label US.
Plasmid Miniprep Kits (50) ACT environmental impact factor label US. Plasmid Miniprep Kits (250) ACT environmental impact factor label US.
Plasmid Miniprep Kits (250) ACT environmental impact factor label US. Plasmid Miniprep Kits (50) ACT environmental impact factor label EU.
Plasmid Miniprep Kits (50) ACT environmental impact factor label EU. Plasmid Miniprep Kits (250) ACT environmental impact factor label EU.
Plasmid Miniprep Kits (250) ACT environmental impact factor label EU. Plasmid Miniprep Kits (50) ACT environmental impact factor label UK.
Plasmid Miniprep Kits (50) ACT environmental impact factor label UK. Plasmid Miniprep Kits (250) ACT environmental impact factor label UK
Plasmid Miniprep Kits (250) ACT environmental impact factor label UK
Die Plasmidaufreinigung mit QIAprep Kits und dem QIAwave Plasmid Miniprep Kit erfolgt nach dem einfachen Prinzip "Binden-Waschen-Eluieren" (siehe Flussdiagramm „ Das QIAprep-Verfahren“). Zunächst werden die Bakterienkulturen lysiert und die Lysate durch Zentrifugation geklärt. Die geklärten Lysate werden dann auf das QIAprep 2.0-Modul aufgebracht, wo die Plasmid-DNA an die Silikamembran adsorbiert. Verunreinigungen werden weggewaschen und die reine DNA wird in einem kleinen Volumen Elutionspuffer oder Wasser eluiert.
Neben der Plasmidaufreinigung aus Escherichia coli können die QIAprep Kits auch zur Aufreinigung von Plasmid-DNA aus Saccharomyces cerevisiae, Bacillus subtilis und Agrobacterium tumefaciens genutzt werden. Sollten Sie Protokolle für diese Anwendungen benötigen, wenden Sie sich bitte an den Technischen Service von QIAGEN oder Ihren Händler vor Ort.
Um Ihr Nutzungserlebnis zu optimieren, können Standardprotokolle mit dem TRACKMAN Connected System und den PIPETMAN M Connected Pipetten, beide von Gilson, durchgeführt werden. Dieses System führt Forschende nicht nur durch die Protokolle, sondern passt auch die Bluetooth-fähigen Pipetteneinstellungen automatisch an. Weitere Informationen herunterladen.
Das QIAprep Spin und das QIAwave Plasmid Miniprep Kit liefern reproduzierbare Ausbeuten an hochreiner DNA, die sich für die meisten Anwendungen eignet, einschließlich:
| Features | Specifications |
|---|---|
| Applications | Fluoreszierende und radioaktive Sequenzierung (einschließlich Kapillarsequenzierung), Ligation, Klonierung, Transformation usw. |
| Processing | Manuell (Vakuum oder Zentrifugation) oder automatisiert |
| Plasmid type | High-Copy, Low-Copy, Cosmid-DNA |
| Culture volume/starting material | 1–10 ml Kulturvolumen |
| Elution volume | 50 µl (Minimum) |
| Technology | Silikatechnologie |
| Time per run or prep per run | <30 Minuten |
| Yield | <20 µg |
| Samples per run (throughput) | 1–24 Proben pro Lauf |
| Number of preps per run | 1–24 Proben pro Lauf |