QIAseq FX DNA Library Kit
For all-enzymatic whole genome and hybrid capture library preparation for Illumina instruments with minimal bias
For all-enzymatic whole genome and hybrid capture library preparation for Illumina instruments with minimal bias
✓ 24/7 automatic processing of online orders
✓ Knowledgeable and professional Product & Technical Support
✓ Fast and reliable (re)-ordering
Cat. No. / ID: 180477
✓ 24/7 automatic processing of online orders
✓ Knowledgeable and professional Product & Technical Support
✓ Fast and reliable (re)-ordering
Our QIAseq FX technology incorporates an all-enzymatic DNA fragmentation into a streamlined, optimized protocol that doesn’t require additional sample clean-up before adapter ligation. A simple, three-reaction protocol enables straightforward automation of library preparation on various liquid-handling platforms, reducing hands-on time and run-to-run variability. The technology is compatible with Illumina sequencing instruments and available in 24-, 96- and 384-sample configurations. Requiring just 2.5 hours for library prep, and with no need for additional expensive DNA shearing equipment, substantial time and costs are saved.
Want to try the QIAseq FX DNA Library UDI Kit for the first time? Request a quote for a trial kit.
Need a quote for your research project or would you like to discuss your project with our specialist team? Contact Us.
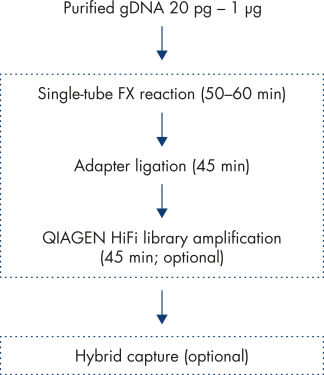
The new QIAseq FX DNA Library Kit takes you from 20 pg – 1 μg genomic DNA to sequencer-ready, amplified libraries in just 2.5 hours. It’s faster and easier to automate than mechanical shearing but still generates the high-quality libraries you need for whole genome sequencing of any organism (see figure: Workflow). The workflow reliably generates reproducible DNA fragments of customizable sizes. (see figures: Customizable fragment sizes and High Reproducibility)
The QIAseq FX DNA Library Kit performs library preparations with minimal bias by using sequence-independent enzymes for fragmentation – delivering results comparable to mechanical shearing. (see figure: Minimal GC bias compared to other enzymatic methods)
QIAseq FX technology with its minimal sequence bias ensures that the majority of genomic targets have very similar total coverage depth. This reduces the need for additional sequencing to bring low-coverage targets up to an interpretable coverage range, saving time and resources. Sequencing libraries generated by the QIAseq FX method have high complexity and low duplicate rates, overcoming the shortcoming commonly associated with other enzymatic fragmentation methods. (see figures: Superior coverage distribution and Lower duplication rate)
Fully functional dual bar-coded adapters and optional high-fidelity library amplification reagents enable the use of QIAseq FX fragmentation and adapter ligation chemistries to generate PCR-free libraries in under two hours total workflow time. Without additional PCR library amplification, the QIAseq FX DNA Library Kit can consistently generate the higher than 2 nM PCR-free library concentration needed for sequencing on an Illumina MiSeq or NextSeq 500 – from as little as 100 ng input DNA. (see figure: No significant differences between coverage of high or low GC genomic regions)
QIAGEN’s QIAseq FX technology incorporates enzymatic DNA fragmentation into a streamlined, optimized protocol that combines fragmentation and adapter ligation in one reaction, saving time and preventing errors. The QIAseq FX kit contains a novel nuclease formulation that digests dsDNA in a random fashion without sequence preference, generating sequencing libraries with minimal bias. The fragment size is tunable simply by changing incubation time of the dsDNA with the nuclease, enabling different sequencing applications. Optimized enzyme and buffer compositions ensure high sequencing library yield. Streamlined library construction protocols also enable straightforward automation of library prep on different liquid-handling platforms.
The QIAGEN QIAseq FX DNA Library Kit provides a fast, fully enzymatic procedure, from DNA fragmentation to NGS library, with no cleanup steps until after adapters have been ligated to the sample DNA.
The QIAseq FX DNA Library Kit consists of three, easy-to-follow steps starting from genomic DNA and ending with sequencer-ready NGS libraries. Beginning with combined DNA fragmentation and end modification, sample fragments are prepared allowing for the binding of dual bar-coded adapters. For samples that are less than 100 ng of input DNA or if large amounts of library DNA are required in later applications, an optional DNA amplification step can be performed with reagents conveniently provided in this kit.
Samples consisting of longer DNA fragments, such as genomic DNA or amplicons from long-range PCR, are first enzymatically sheared into smaller fragments. The median fragment size is dependent on the applications and sequencing read length, and can be adjusted by varying the DNA fragmentation reaction conditions. The fragmented DNA is directly end-repaired, and an 'A’ is added to the 3’ ends during the FX reaction – making the DNA fragments ready for adapter ligation. Following this step, platform-specific adapters are ligated to both ends of the DNA fragments. These adapters contain sequences essential for binding dual bar-coded libraries to a flow cell for sequencing, allowing for PCR amplification of adapter-ligated fragments and for binding of standard Illumina sequencing primers.
To ensure maximum yields from limited amounts of starting material, a high-fidelity amplification step can be performed using the reagents included in the QIAseq FX DNA Library Kit. The proprietary HiFi PCR Master Mix can evenly amplify DNA regions with vastly different GC contents, minimizing sequencing bias caused by PCR.
Dual bar-coded, plate-format adapters are included with the QIAseq FX DNA Library Kit. Each well contains a a unique combination of two identification bar codes. QIAseq FX kits support up to 24-plex,96-plex or 384-plex pooling prior to sequencing.
Following library construction, the reaction cleanup and removal of adapter dimers can be achieved by using QIAseq Beads, enabling easy automation on various high throughput automation platforms.
Normalization
The QIAseq Library Normalizer seamlessly integrates with the QIAseq FX DNA Library Prep Kit, removing the need for tedious qPCR and manual dilution of libraries before pooling. Normalized libraries are ready-to-sequence dsDNA at approximately 4 nmol/L.