Products
Features
- Unique Molecular Indices (UMI) ensure accurate sequencing results
- Online data analysis through GeneGlobe
- Includes QIAseq Beads for reaction cleanup
- Automation-friendly protocol for human or mouse samples
- QIAseq Sample Indices ordered separately to multiplex up to 384 samples
Product Details
The QIAseq Immune Repertoire RNA Library Kits use Unique Molecular Indices (UMI) with gene-specific primers to target specific RNAs for NGS sequencing. Each unique panel is carefully designed and laboratory-verified for sequencing performance with a UMI-aware alignment software for maximum sequencing performance and accurate results. The Human and Mouse T-cell Receptors Panel is used for sequencing the V(D)J region of the alpha, beta, delta and gamma genes, including the CDR3 regions. Online analysis through the GeneGlobe Data Analysis Center provides key sequencing QC metrics, as well as the frequency and identity of each clonotype sequenced.
This kit has been upgraded to the QIAseq Targeted RNA-seq Panel for T-cell Receptor. We recommend starting new projects with the upgraded kits.
See figures
Performance
Comprehensive view of the T-cell immune repertoire
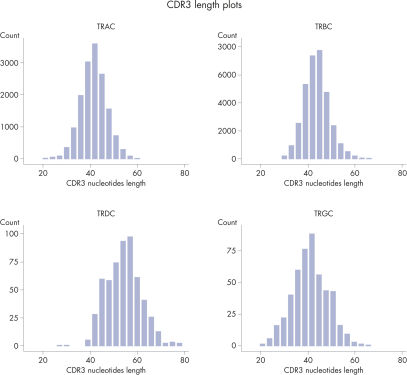
The heatmaps allow for easy identification of enriched clonotypes across the sample. This figure shows the major clonotype of the Jurkat cell, as well as the diversity of the PBMC background. The data analysis included with the purchase of the QIAseq Immune Repertoire T-cell receptor panels includes an online portal that seamlessly integrates with Illumina BaseSpace and provides primary read mapping, UMI demultiplexing and reports on sequencing performance, TCR chain usage, CDR3 peptide sequence and length distributions, together with rarefaction and V/D/J usage heat maps.Sensitive to at least 0.01%
RNA from Jurkat cells was spiked into RNA extracted from peripheral blood mononuclear cells (PBMCs; Precision Medicine) at 10%, 1%, 0.1% and 0.01% and used to make an RNA-seq library. Table 1 shows the number of raw reads and the demultiplexed unique captures (UMIs) per Jurkat TCR-alpha and TCR-beta clonotype. Even when present at only 0.01%, the Jurkat RNA is readily quantifiably identified. For data analysis, UMIs and Raw Reads are used to ensure high precision around each clonotype sequence identified.
| Chain | % Jurkat cells | Rank | Reads | UMIs |
|---|---|---|---|---|
| TCR-alpha | 10 | 1 | 751,749 | 107,150 |
| 1 | 1 | 146,959 | 20,692 | |
| 0.1 | 1 | 10,708 | 1,742 | |
| 0.01 | 10 | 1,306 | 217 | |
| TCR-beta | 10 | 1 | 383,594 | 40,943 |
| 1 | 1 | 5,920 | 7,541 | |
| 0.1 | 2 | 5,401 | 620 | |
| 0.01 | 61 | 457 | 60 |
See figures
Principle
The QIAseq Immune Repertoire RNA Library Kit relies on a highly efficient, TCR-specific cDNA synthesis reaction, ligation of sample index adapters containing UMIs and TCR gene-specific primer enrichment for sensitive TCR clonotype and diversity assessment. Each kit contains species-specific TCR reverse transcriptase and enrichment panel primers, together with QIAseq reaction cleanup beads and library reagents. The QIAseq Immune Repertoire RNA Library Kit is designed to enrich TCR α, β, γ and σ subunits using 10–1000 ng RNA from human or mouse samples.
Procedure
The QIAseq Immune Repertoire RNA Library Kit relies on a highly efficient, TCR-specific cDNA synthesis, TCR gene-specific primer enrichment and molecular indexing for accurate and sensitive TCR clonotype and diversity assessment (see figure " QIAseq Immune Repertoire RNA Library workflow"). TCR reverse transcriptase and enrichment panel primers are provided, together with library reagents.
cDNA synthesis
RNA samples are first reverse transcribed into cDNA with TCR-specific RT primers. Subsequently, second-strand synthesis occurs, which generates double-stranded cDNA (ds-cDNA). This ds-cDNA is then end-repaired and A-tailed in a single-tube protocol.
UMI assignment
Prior to target enrichment and library amplification, each original cDNA molecule is assigned a UMI by ligating an adapter containing a 12-base fully random sequence (i.e., the UMI) to the ds-cDNA. Statistically, this process provides 4^12 possible indices per adapter, and each DNA molecule in the sample receives a unique UMI sequence. In addition, this ligated adapter also contains the first sample index.
Target enrichment and final library construction
Following UMI assignment, target enrichment is performed to ensure that TCR cDNA molecules are sufficiently enriched in the sequenced library. For enrichment, ligated cDNA molecules are subjected to targeted PCR using one TCR constant-region-specific primer and one universal primer complementary to the adapter. A universal PCR is ultimately carried out to amplify the library and introduce platform-specific adapter sequences, as well as additional sample indices.
See figures
Applications
Supporting data and figures
CDR3 Length Plots are Shown for Each Receptor in from a Single Sample.