✓ 24/7 automatic processing of online orders
✓ Knowledgeable and professional Product & Technical Support
✓ Fast and reliable (re)-ordering
QIAseq FastSelect –rRNA Worm Kit (24)
Cat. No. / ID: 333242
✓ 24/7 automatic processing of online orders
✓ Knowledgeable and professional Product & Technical Support
✓ Fast and reliable (re)-ordering
Features
- Compatible with QIAGEN, Illumina, NEB and KAPA stranded RNA-seq library kits
- Single reagent for Caenorhabditis elegans (worm )samples
- High-performance rRNA and/or globin removal in just 14 minutes
- Only one pipetting step – combine QIAseq FastSelect reagent with RNA and incubate
- No extra cleanup steps or NGS library protocol changes
Product Details
QIAseq FastSelect –rRNA Worm Kits use a novel method to remove highly abundant RNA that is of low scientific value from your RNA-seq libraries. Researchers using RNA-seq for whole transcriptome analysis can use QIAseq FastSelect –rRNA Worm Kits to remove all annotated rRNAs in RefSeq and Ensembl for Caenorhabditis elegans (worm). Depending on rRNA sequence homology, QIAseq FastSelect −rRNA Worm will work on additional worm species (dependent upon target rRNA sequence homology with C. elegans).
Analyze RNA-seq data with ease using the GeneGlobe-integrated RNA-seq Analysis Portal – an intuitive, web-based data analysis solution created for biologists and included with this QIAseq Kit.
We recommend using QIAseq Stranded RNA Library Kits for robust strand-specific RNA-seq library preparation for high-quality and highly fragmented (FFPE) RNA. QIAseq Stranded RNA Library Kits utilize unique dual indexing (UDIs) which enables multiplexing of up to 384 samples per flow cell lane on Illumina NGS instruments and mitigates index hopping on Illumina NovaSeq and Illumina patterned flow cells.
Design your own custom QIAseq FastSelect pools to remove any RNAs you wish from your RNA-seq library – take a look at our QIAseq FastSelect Custom RNA Removal Kits.
Want to try the QIAseq FastSelect –rRNA Worm Kit for the first time? Request a trial kit to evaluate.
Performance
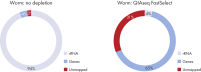
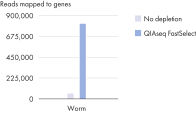
QIAseq FastSelect –rRNA Worm Kits provide highly efficient removal of rRNA (figure QIAseq FastSelect –rRNA Worm Kits provide highly efficient removal of rRNA) and consistent read mapping (figures QIAseq FastSelect –rRNA Worm exon/intron/intergenic mapping, QIAseq FastSelect ‒rRNA Worm dramatically shifts read allocation from rRNA to genes and QIAseq FastSelect ‒rRNA Worm dramatically shifts read allocation from rRNA to genes: bar chart).
Principle
Removing highly expressed, but biologically unimportant RNA transcripts makes NGS more efficient and enables higher sample throughput with higher sensitivity. Furthermore, removal of unwanted RNA species from full length and fragmented RNA samples can be particularly challenging and can result in suboptimal performance.
QIAseq FastSelect –rRNA Worm Kits are designed for quick, efficient removal of cytoplasmic and mitochondrial worm ribosomal RNA from total RNA during NGS RNA library preparation. QIAseq FastSelect seamlessly integrates with your existing RNA stranded library preparation workflow for RNA removal in a single, 14-minute inline step. Prior to RNA heat fragmentation (which is optional and dependent upon the library preparation kit and sample type), QIAseq FastSelect removal reagent is directly combined with total RNA and the library preparation-specific buffers. After fragmentation, the reaction temperature is stepwise cooled to room temperature and the remaining library preparation steps are completed. There is no need to perform any type of enrichment on the total RNA samples. QIAseq FastSelect –rRNA Worm Kits ensure consistently high performance with RNA amounts ranging from as little as 1 ng up to 1 μg. QIAseq FastSelect can be used with RNA from fresh samples from many species of worm (dependent upon target rRNA sequence homology with C. elegans), and delivers reliable rRNA removal and high reproducibility in downstream applications.
Procedure
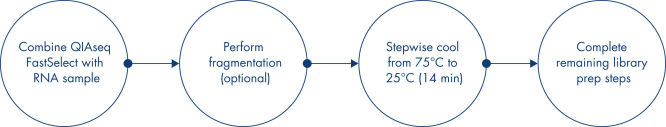
Most RNA removal or depletion strategies associated with RNA-seq library construction are sample pre-treatment strategies involving hybrid-capture or enzymatic removal of unwanted RNA. Our unique QIAseq FastSelect procedure is compatible with QIAGEN, Illumina, KAPA and NEB and other RNA library kits and provides complete rRNA removal in a single, 14-minute inline step (see figure QIAseq FastSelect Kit workflow). This is dramatically faster than alternative RNA depletion kits, which require pre-treatment protocols involving more than 25 steps and 2 hours to complete.
Simply add QIAseq FastSelect reagent to the RNA sample, perform fragmentation (if required), stepwise cool the reaction from 75°C to 25°C for 14 minutes and then complete the remaining library preparation steps. QIAseq FastSelect works with or without RNA fragmentation, providing the flexibility to use fragmented RNA from worm samples or high-quality RNA as part of a standard RNA-seq library construction workflow.
See figures
Applications
QIAseq FastSelect delivers rapid, reliable RNA removal from full length or fragmented RNA samples from many species of worm (dependent upon target rRNA sequence homology with C. elegans). QIAseq FastSelect –rRNA Worm Kits are available in a variety of different formats and sizes to suit your specific applications.
Supporting data and figures
QIAseq FastSelect Kit workflow