QIAsymphony PAXgene Blood ccfDNA Kit (CE-IVD) (192)
Cat. No. / ID: 768566
Features
- CE Marked for In Vitro Diagnostic (IVD) use
- Optimized binding chemistry and input volumes for PAXgene Blood ccfDNA Tubes (CE-IVD)
- Prefilled reagent cartridges with barcode for safety and ease of use
- Primary tube handling protocols allow centrifuged PAXgene Blood ccfDNA Tubes (CE-IVD) to be placed directly on the QIAsymphony SP instrument
- The PAXgene Blood ccfDNA Tube (CE-IVD), the QIAsymphony PAXgene Blood ccfDNA Kit (CE-IVD), and QIAsymphony Instrument (CE-IVD) are all CE marked for In Vitro Diagnostic Use according to Regulation (EU) 2017/746 on In Vitro Diagnostic Medical Devices
Product Details
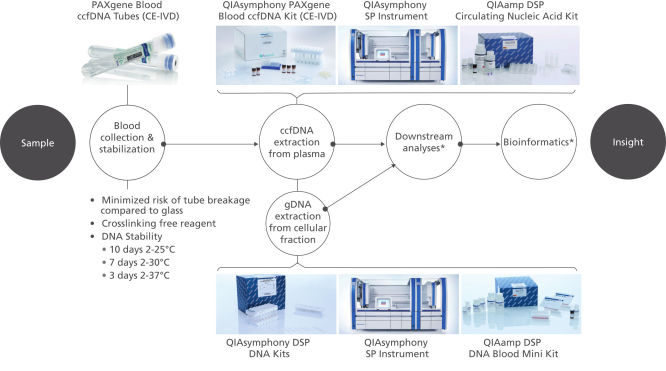
The QIAsymphony PAXgene Blood ccfDNA Kit (CE-IVD), to be used with the QIAsymphony SP instrument, is intended for automated isolation and purification of circulating cell-free DNA (ccfDNA) from plasma generated from human venous whole blood collected in the PAXgene Blood ccfDNA Tube (CE-IVD).
The QIAsymphony PAXgene Blood ccfDNA Kit (CE-IVD) utilizes magnetic-particle technology for automated isolation and purification of ccfDNA from human plasma.
The QIAsymphony PAXgene Blood ccfDNA Kit (CE-IVD) is for in vitro diagnostic use and to be used by professional users, such as technicians and physicians who are trained in molecular biology techniques.
The PAXgene Blood ccfDNA Tube (CE-IVD), the QIAsymphony PAXgene Blood ccfDNA Kit (CE-IVD), and QIAGEN QIAsymphony instrument (CE-IVD) are all CE marked for In Vitro Diagnostic User according to Regulation (EU) 2017/746 on In Vitro Diagnostic Medical Devices. (See "QIAsymphony PAXgene Blood ccfDNA workflow (CE-IVD).").
Plasma generated from whole blood collected into PAXgene Blood ccfDNA Tubes (CE-IVD) can be used to process ccfDNA automated with the QIAsymphony PAXgene Blood ccfDNA Kit (CE-IVD), or manually with the QIAamp DSP Circulating Nucleic Acid Kit. The nucleated cellular fraction or buffy coat remaining after removal of the plasma can be processed for gDNA automated with the QIAsymphony DSP DNA Mini and Midi Kits, or manually with the QIAamp DSP DNA Blood Mini Kit.
See figures
Performance
The PAXgene Blood ccfDNA workflow (CE-IVD) consists of a blood collection tube, the PAXgene Blood ccfDNA Tube (CE-IVD), and a circulating cell-free DNA (ccfDNA) purification kit, the QIAsymphony PAXgene Blood ccfDNA Kit (CE-IVD), for automated extraction of ccfDNA on the QIAsymphony SP Instrument. Together, the tube and kit constitute a complete in vitro diagnostic medical device preanalytical workflow. (See " PAXgene Blood ccfDNA workflow (CE-IVD).").
The QIAsymphony PAXgene Blood ccfDNA Kit magnetic-particle technology enables purification of high-quality ccfDNA that is free of proteins, nucleases, and other impurities.
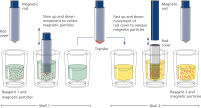
The QIAsymphony SP instrument performs all steps of the purification procedure. The technology combines the speed and efficiency of anion exchange-based nucleic acid purification with the convenient handling of magnetic particles. The purification procedure is designed to ensure safe and reproducible handling of potentially infectious samples. Onboard measures minimize cross-contamination. Up to 96 samples, in batches of 24, are processed in a single run (See "Schematic diagram of the QIAsymphony SP instrument principle.").
If similar input volumes of blood are used, ccfDNA yields achieved with the QIAsymphony PAXgene Blood ccfDNA Kit (CE-IVD) are comparable to manual extraction with the QIAamp DSP Circulating Nucleic Acid Kit (See " Relative yield for ccfDNA from PAXgene Blood ccfDNA Tube (CE-IVD) plasma processed automated or manually compared to EDTA tube plasma at Day 0.").
Four different protocols were established. In the standard versions, sample input volumes of 2.4 mL or 4.8 mL plasma can be selected. A “less sample” function, an integrated part of the protocol, allows the transfer of lower plasma volumes with minimum input volumes of 1.6 mL or 4.1 mL respectively (See " ccfDNA extraction from different plasma volumes with the standard protocols.").
The Primary Tube handling protocols allow direct placement of the PAXgene Blood ccfDNA Tube (CE-IVD) onto the QIAsymphony SP instrument. Primary handling saves time, reduces costs and biohazard waste, and minimizes misidentification and risk of exposure. Primary Tube handling protocols are available for sample input volumes of 2.4 mL or 4.0 mL plasma (See " Primary tube handling protocols for sample input volumes of 2.4 mL and 4.0 mL plasma.").
Preanalytical steps including handling, storage, processing and documentation of verification and validation studies of QIAsymphony PAXgene Blood ccfDNA Kit (CE-IVD) were conducted in accordance with ISO 20186-3:2019: Molecular in vitro diagnostic examinations — Specifications for pre-examination processes for venous whole blood — Part 3: Isolated circulating cell free DNA from plasma. The QIAsymphony PAXgene Blood ccfDNA workflow (CE-IVD) covers the whole liquid biopsy preanalytical workflow from blood collection to automated ccfDNA extraction from plasma with high intermediate, inter-run, and within-run precision (See " Evaluation of intermediate, inter-run precision (repeatability), and within-run workflow precision.").
The purified ccfDNA eluates can be stored for long-term at −20°C or −80°C, including at least three freeze and thaw cycles (for latest update see technical note ´Eluate Stability Study.´) and are compatible with downstream analytical PCR assays, including user-validated methylation-based PCR, and next-generation sequencing (NGS) molecular test methods.
See figures
 Relative yield for ccfDNA from PAXgene Blood ccfDNA Tube (CE-IVD) plasma processed automated or manually compared to EDTA tube plasma at Day 0.
Relative yield for ccfDNA from PAXgene Blood ccfDNA Tube (CE-IVD) plasma processed automated or manually compared to EDTA tube plasma at Day 0.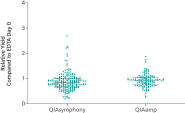 ccfDNA extraction from different plasma volumes with the standard protocols.
ccfDNA extraction from different plasma volumes with the standard protocols.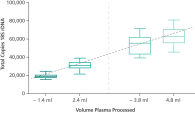 Primary tube handling protocols for sample input volumes of 2.4 mL and 4.0 mL plasma with the QIAsymphony PAXgene Blood ccfDNA Kit (CE-IVD).
Primary tube handling protocols for sample input volumes of 2.4 mL and 4.0 mL plasma with the QIAsymphony PAXgene Blood ccfDNA Kit (CE-IVD). Evaluation of intermediate, inter-run precision (repeatability), and within-run workflow precision.
Evaluation of intermediate, inter-run precision (repeatability), and within-run workflow precision.
Principle
Circulating cell-free DNA (ccfDNA) is present in plasma usually as short fragments (<1000 bp). The concentration of ccfDNA in plasma is usually low (can range from 1–100 ng/mL) and varies considerably between individuals.
The PAXgene Blood ccfDNA Tube (CE-IVD) is a plastic, closed, evacuated tube for the collection, anticoagulation, transport, and storage of 10 mL human whole blood samples. The additive in the tube prevents the release of intracellular DNA into the plasma and maintains constant ccfDNA levels for 10 days at temperatures up to 25°C, 7 days at temperatures up to 30°C, or 3 days at temperatures up to 37°C.
Plasma generated from whole blood collected into PAXgene Blood ccfDNA Tubes (CE-IVD) can be used to process ccfDNA automated with the QIAsymphony PAXgene Blood ccfDNA Kit (CE-IVD) on the QIAsymphony SP instrument.
The PAXgene Blood ccfDNA Tube (CE-IVD) with the QIAsymphony PAXgene Blood ccfDNA Kit (CE-IVD) and QIAsymphony instrument (CE-IVD) have been validated as an integrated workflow.
Procedure
Blood is collected under a standard phlebotomy protocol into the PAXgene Blood ccfDNA Tube (CE-IVD) (see PAXgene Blood ccfDNA Tube (CE-IVD) Instructions for Use).
In the standard protocols, plasma is generated by centrifugation at room temperature (15–25°C) for 15 minutes at 1,600–3,000 × g. Plasma is removed after the first centrifugation into a secondary tube, centrifuged again for 10 minutes at 1,600-3,000 × g, transferred into a 14 mL 17 × 100 mm polystyrene, round-bottom tube, placed into a tube carrier, and loaded into the sample input drawer of the QIAsymphony SP instrument.
Sample input volumes of 2.4 mL or 4.8 mL plasma can be selected, but due to void volumes of an additional 0.4 mL and 0.5 mL, a minimum of 2.8 mL and 5.3 mL plasma respectively must be placed onto the instrument. If lower plasma volumes than 2.8 mL or 5.3 mL are available, the “Less Sample mode”, an integrated part of the protocol function, allows processing of lower plasma volumes by the instrument. In the result file the discrepancy between the regular and the transferred plasma volume is documented. The minimum plasma input volumes to enable “Less Sample mode” are 1.6 mL or 4.1 mL.
As an alternative to the standard protocols, the primary tube handling function allows the tube to be directly placed on the QIAsymphony SP instrument after the first centrifugation. In order to use the primary tube handling function, centrifugation for 15 minutes at 3,000 × g is mandatory. Plasma volume in each tube after removal from the centrifuge bucket are conveniently quantified with the PAXgene Blood ccfDNA Purification Protocol Selection Tool provided as a kit content to select one of the Primary Tube handling protocols for sample input volumes of 2.4 mL or 4.0 mL plasma (See " Plasma volume determination using the PAXgene Blood ccfDNA Purification Protocol Selection Tool.").
On the QIAsymphony SP instrument, plasma proteins are digested by proteinase K while ccfDNA binds to the surface of magnetic beads. Three wash steps ensure contaminant removal.
Finally, ccfDNA is eluted from the magnetic particles and is ready for use in downstream IVD applications.
See figures
Applications
ccfDNA purified from plasma generated from whole blood collected in PAXgene Blood ccfDNA Tubes (CE-IVD) using the QIAsymphony PAXgene Blood ccfDNA Kit (CE-IVD) and the QIAsymphony SP instrument is ready for use in a wide range of downstream applications, including:
- PCR, including digital, multiplex and quantitative real-time PCR
- Pharmacogenomic studies
- SNP genotyping
- Next-generation sequencing
- Methylation assays
PAXgene Blood ccfDNA Tube (CE-IVD) and QIAsymphony PAXgene Blood ccfDNA Kit (CE-IVD) are for in vitro diagnostic use.
Supporting data and figures
QIAsymphony PAXgene Blood ccfDNA workflow (CE-IVD).
PAXgene Blood Tube (CE-IVD) and QIAsymphony PAXgene Blood ccfDNA Kit (CE-IVD) are CE Marked for In Vitro Diagnostic use according to EU Regulation on in-vitro diagnostic medical devices (REGULATION (EU) 2017/746).
Plasma generated from whole blood collected into PAXgene Blood ccfDNA Tubes (CE-IVD) can be used to process ccfDNA automated with the QIAsymphony PAXgene Blood ccfDNA Kit (CE-IVD) on the QIAsymphony SP instrument.
*Complete workflow CE-IVD only when used in combination with products for In Vitro Diagnostic use.
Specifications
| Features | Specifications |
|---|---|
| Features | Two reagent cartridges for 96 plasma samples per cartridge |
| Technology | Magnetic particle technology |
| Input volume | Primary Tube handling: 2.4 mL or 4.0 mL plasma; Standard protocols: 2.4 mL or 4.8 mL plasma |
| Elution volume | 60 μL |
| Sample type | Plasma from venous whole blood collected into PAXgene Blood ccfDNA Tubes (CE-IVD) |
| Time per run | 96 samples with 4.8 mL plasma in ≤ 6 hours (< 5 h 40 m, including plasma preparation, instrument setup and run) |
| Throughput | 192 samples per working day (8h) and instrument: 2 x 96 samples from plasma generation to the start of the second run (overnight) with 96 samples |
| Processing | Automated: QIAsymphony SP Instrument; Standard protocols: Plasma after second centrifugation transferred into secondary tube; Primary Tube handling: PAXgene Blood ccfDNA Tube (CE-IVD) placed on the instrument after one centrifugation |
| Shelf life | Open reagent cartridge: 4 weeks; See expiration date printed on the label |
| Special feature | 4 runs per reagent cartridge possible |
| Stabilization | Eluate stability: Long-term storage frozen at −20°C or −80°C (for latest update see technical note ´Eluate stability´) including at least 3 freeze/thaw cycles |