QIAcuityDx Four
Cat. No. / ID: 911060
Features
- For precise and multiplexed quantification results for rare mutation detection, copy number variation (CNV), gene expression studies, gene-editing analysis, and many more
- Integrates a standard dPCR workflow into a walk-away automated platform
- Fully automated processing of QIAcuityDx Nanoplates
- Minimal hands-on time
- Dual mode software: Utility (open) and IVD (closed) modes
- Explore more than 200 dPCR assays on GeneGlobe for use in utility mode for research purposes
Product Details
The QIAcuityDx performs fully automated processing of QIAcuityDx nanoplates , including all necessary steps of nanoplate partitioning, thermocycling and image analysis. Depending on the plate type, up to 96 samples per plate can be analyzed. For diagnostic applications, the QIAcuityDx Nanoplate 26K is available with 24 wells. A total of four nanoplates can be simultaneously processed, with continuous loading possible. The QIAcuityDx Control Software controls all integrated modules, including a robotic gripper for nanoplate handling, a partitioning module, a PCR thermocycler and a fluorescence imaging module.
Performance
The QIAcuityDx Digital PCR System makes absolute quantification accessible for all laboratories. The walk-away automation integrates and streamlines the entire dPCR workflow of partitioning, thermocycling and imaging into a single instrument with minimal hands-on time. No change in plate handling is required when coming from qPCR, assuring fast assay setup and quick results in approximately two hours for the first plate.
QIAcuityDx Instruments – features and specifications
| Operating modes | IVD (closed) Utility (open) |
| Plates processed per run | 4 |
| Plate loading style | Continuous loading |
| Detection channels (multiplexing) | 5 |
| Time to result of first plate | 120 minutes |
| Each subsequent plate | 80 minutes |
| Throughput | 8 plates –192 samples Dx mode (Dx 24 well, 26,000 partition plate)* 8 plates – 768 samples Utility Mode (Research Use: 96 well, 8,000 partition plate)* |
| Dimensions | Width 60 cm (23.6 in.) / Height 58 cm (22.8 in.) / Depth 65 cm (25.6 in.) |
*Based upon an 8-hour working day
Principle
The QIAcuityDx is designed as a walk-away instrument that integrates and automates all plate processing steps. Only the nanoplate preparation must be done manually before starting the run. If this preparation is done and the experiment is set up, the nanoplate must be placed in a free plate slot of the instrument tray. By reading the barcode of the plate, the instrument links the nanoplate to the experimental parameters previously defined in the software. After initiating the run, all further steps are performed, fully automated, by the instrument.
Procedure
Just like in a qPCR workflow, sample preparation includes the transfer of master mix, probes and primers to any QIAcuity plate type* followed by the addition of samples. The system integrates partitioning, thermocycling and imaging into a single, fully automated instrument that achieves sample to result in under 2 hours. One can perform analysis on the Software Suite, providing the concentration in copies per microliter of your target sequence as well as for quality control such as positive samples or NTC. This analysis can also be extended to remote computers within the same local area network (LAN).
* An IVD variant of the 24-well, 26,000 partition nanoplate will be available for use with QIAcuityDx, in either Utility Mode or IVD Mode. Utility Mode, intended for translational research or for laboratories to develop their own developed tests workflow, will allow the use of QIAcuity research use plate variants.
Applications
The QIAcuityDx may be used for a wide range of applications, including, but not limited to, oncology and infectious disease testing. From a biological risk perspective, the QIAcuityDx is a ‘closed’ system once a top-seal is applied to a nanoplate, thus significantly reducing the risk of instrument contamination and potential user infection.
The QIAcuityDx instrument, together with the QIAcuityDx Nanoplates and the QIAcuityDx PCR master mixes and assays, enable clinical dPCR applications, including:
- Rare mutation detection and quantitation
- Copy number variation analysis
- Gene expression analysis
- Pathogen detection and quantitation
- Genotyping
Software
The QIAcuityDx Software Suite provided with the instrument and installed on a separate computer controls one or multiple QIAcuityDx instruments, either connected directly to one instrument or using an existing local area network (LAN). Using the QIAcuityDx Software Suite, dPCR experiments, samples and reaction mixes can be defined, assigned to Nanoplates, and transferred to the QIAcuityDx instrument. After the run, data can be analyzed, reports can be created and data can be exported for external analysis. The software provides multiple template functionalities to make repeating plate layouts or plate run parameters easily accessible, further transforming your dPCR experience.
All IVD assays are provided with a unique Software Assay Package (SAP), which contains locked run and analysis parameters to be used in IVD mode. This eliminates the risk of run setup errors and provides an automated analysis that delivers easily interpretable results (that is, target positive rather than a numerical value).
When integrated into a local area network, the computer that hosts the QIAcuityDx Software Suite functions as a server accessible via LAN to other computers serving as clients. This enables multiple users to access the software from other rooms or offices and analyze data via a standard browser without having to install the software on multiple computers or access and exchange data via internet connections.
Services
Enhance instrument performance with QIAGEN service solutions. Choose from our comprehensive range of service products for the QIAcuityDx, ensuring optimal accuracy, precision, and reproducibility in line with the manufacturer’s specifications. Explore our specific service agreements for more details.
Supporting data and figures
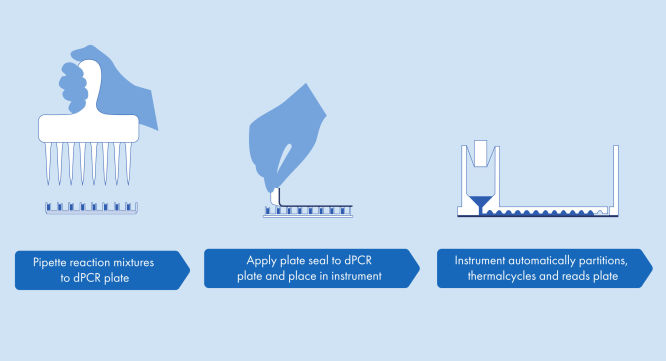
A simple and rapid plate-based workflow
Partitioning, thermocycling and imaging are all integrated into a single fully automated instrument, delivering results in under two hours. Nanoplate formats are amenable to front-end automation for sample preparation, further reducing hands-on time.
Service Plans
QIAcuityDx, Premium Agreement
Cat. No. / ID: 9245980
QIAcuityDx, Basic Agreement
Cat. No. / ID: 9245982
QIAcuityDx, Core Agreement
Cat. No. / ID: 9245983