Products
Features
- Designed for 100-ring disc RT2 Profiler / RT2 lncRNA PCR Arrays
- Available with ROX, fluorescein, or without dye
- Well-suited for use with self-designed PCR assays
- Exceptional qPCR performance in instruments with fast cycling conditions
Product Details
RT2 SYBR Green FAST Mastermixes are specially formulated for use with the 100-ring disc format of the RT2 Profiler and RT2 lncRNA PCR arrays on the Rotor-Gene Q. This mastermix is suited for use with self-designed qPCR assays on other types of real-time PCR instruments with fast cycling conditions. RT2 SYBR Green FAST Mastermixes are available with ROX, fluorescein, or without reference dyes.
Performance
RT² SYBR Green FAST Mastermixes contain all of the optimized reagents and buffers needed for SYBR Green based real-time PCR with RT2 qPCR Primer Assays and RT2 Profiler PCR Arrays on all real-time PCR instruments.
The high-performance real-time PCR enabled by RT² SYBR Green FAST Mastermixes demonstrates high amplification efficiencies (see figure " High amplification efficiency over a wide dynamic range") and high levels of sensitivity and specificity (see figure " Tighter control of polymerase activity yields greater specificity").
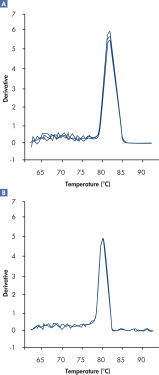
In addition, dissociation curves obtained using RT2 SYBR Green FAST Mastermixes have lower background and a sharper shape compared with dissociation curves generated by regular cycling conditions (see figure " Sharper dissociation curves with RT2 SYBR Green Fast Mastermix").
See figures
Principle
RT2 SYBR Green FAST Mastermixes contain real-time PCR buffer, a high-performance, HotStart DNA Taq polymerase, nucleotides, and SYBR Green dye. Some mastermixes contain either fluorescein or ROX dye for optimization of the instrument optics. The chemically-modified and tightly controlled HotStart enzyme uniquely provides accurate SYBR Green results by preventing the amplification of primer–dimers and other nonspecific products. RT2 SYBR Green FAST Mastermixes are optimized to use a 10-second denaturation step and 30-second annealing/extension step PCR protocol, without compromising specificity or sensitivity.
Mastermixes without ROX or fluorescein
RT2 SYBR Green FAST Mastermix is highly suited for real-time PCR applications using SYBR Green-based detection on instrumentation not requiring a reference dye, including Bio-Rad models CFX96, CFX384; Bio-Rad/MJ Research Chromo4, DNA Engine Opticon, DNA Engine Opticon 2; Roche LightCycler 480 (96-well and 384-well); Eppendorf Mastercycler ep realplex without ROX filter set; and Cepheid SmartCycler.
Mastermixes with fluorescein
RT2 SYBR Green Fluor FAST Mastermix is highly suited for real-time PCR applications using SYBR Green-based detection on instrumentation that uses fluorescein as a reference dye, including the Bio-Rad models iCycler, iQ5, MyiQ, and MyiQ2.
Mastermixes with ROX
RT2 SYBR Green ROX FAST Mastermix is highly suited for real-time PCR applications using SYBR Green-based detection on instrumentation that uses ROX as a reference dye, including QIAGEN’s Rotor-Gene Q; Applied Biosystems models 5700, 7000, 7300, 7500 (Standard and Fast), 7700, 7900HT (Standard and Fast 96-well block, 384-well block), StepOnePlus, ViiA 7 (Standard and Fast 96-well block, 384-well block); Eppendorf Mastercycler ep realplex with or without ROX filter set; Stratagene models Mx3000P, Mx3005P, Mx4000; and Takara TP-800.
Applications
Supporting data and figures
Sharper dissociation curves with RT2 SYBR® Green FAST Mastermix.
A positive PCR control was amplified on the ABI 7500 qRT-PCR instrument using [A] regular cycling and RT² SYBR Green Mastermix or [B] fast cycling with RT² SYBR Green FAST Mastermix. The dissociation curve obtained with RT² SYBR Green FAST Mastermix has lower background and a sharper shape.